Photo AI
Last Updated Sep 24, 2025
Physical Changes Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Physical Changes quickly and effectively.
221+ students studying
Physical Changes
Understanding physical changes is crucial in the study of both chemistry and physics. Such changes affect a substance's form or appearance while maintaining its chemical composition. Comprehending these transformations and their characteristics is fundamental to grasping core concepts in chemistry.
Definition
Physical Changes: Physical changes involve modifying the form or appearance of a substance without altering its chemical composition.
Physical Changes: Modifications in form or appearance without altering chemical composition.
Key Characteristics
-
Reversibility:
- Physical changes are commonly reversible. An example is water, which can freeze and subsequently melt back into a liquid.
- Some changes are not reversible. For instance, grinding a substance might permanently alter its form.
-
Retention of Chemical Identity:
- The chemical identity of a substance remains unchanged during these changes.
infoNoteReversibility and the preservation of chemical identity are critical aspects when analysing physical changes.
Illustrative Examples
-
Melting Ice:
- Ice changes from solid to liquid upon melting, with no new substances formed.
- Reversible by refreezing the water into ice.
-
Boiling Water:
- Water transitions from liquid to gas (steam). It remains H₂O in all states.
- It is purely a physical change, involving no new substances.
-
Dissolving Sugar:
- Sugar dissolves in water but maintains its chemical integrity.
- Sugar can return to its crystalline form upon evaporation.
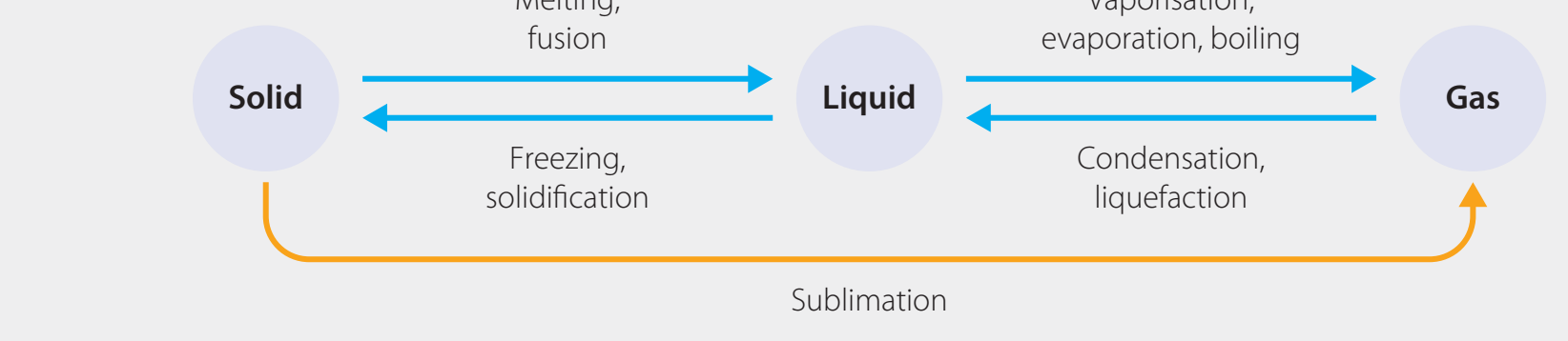
Detailed Examples of Physical Changes
Changes of State
-
Melting: Solid transforms into a liquid (e.g., ice to water).
- Energy Input: Requires energy to break molecular bonds.
-
Freezing: Liquid becomes solid (e.g., water to ice).
- Energy Release: Allows molecules to form a structured arrangement.
-
Boiling: Liquid changes to gas (e.g., water into steam).
- Energy Requirement: Overcomes intermolecular forces.
-
Condensation: Gas transitions back to liquid while releasing energy.
Energy changes are vital in these transitions. Melting and boiling necessitate energy input, whereas freezing and condensation involve energy release.
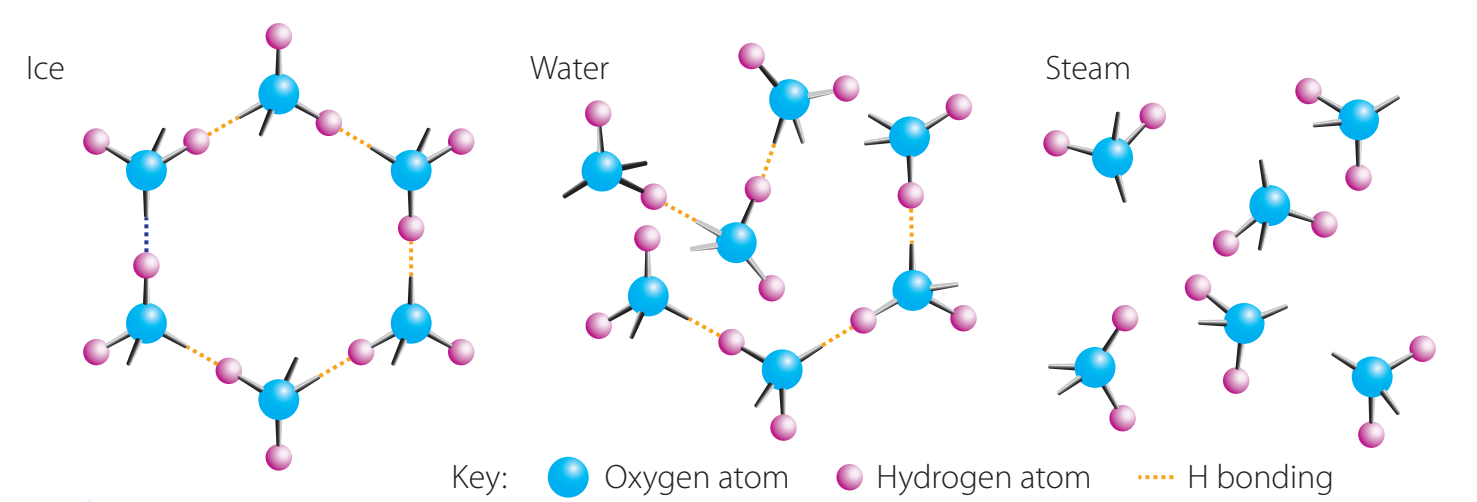
Dissolution
- Sugar and Salt: These dissolve in water and retain their chemical identity.
- Salt: Dissociates into sodium and chloride ions surrounded by water.
- Sugar: Disperses evenly at the molecular level.
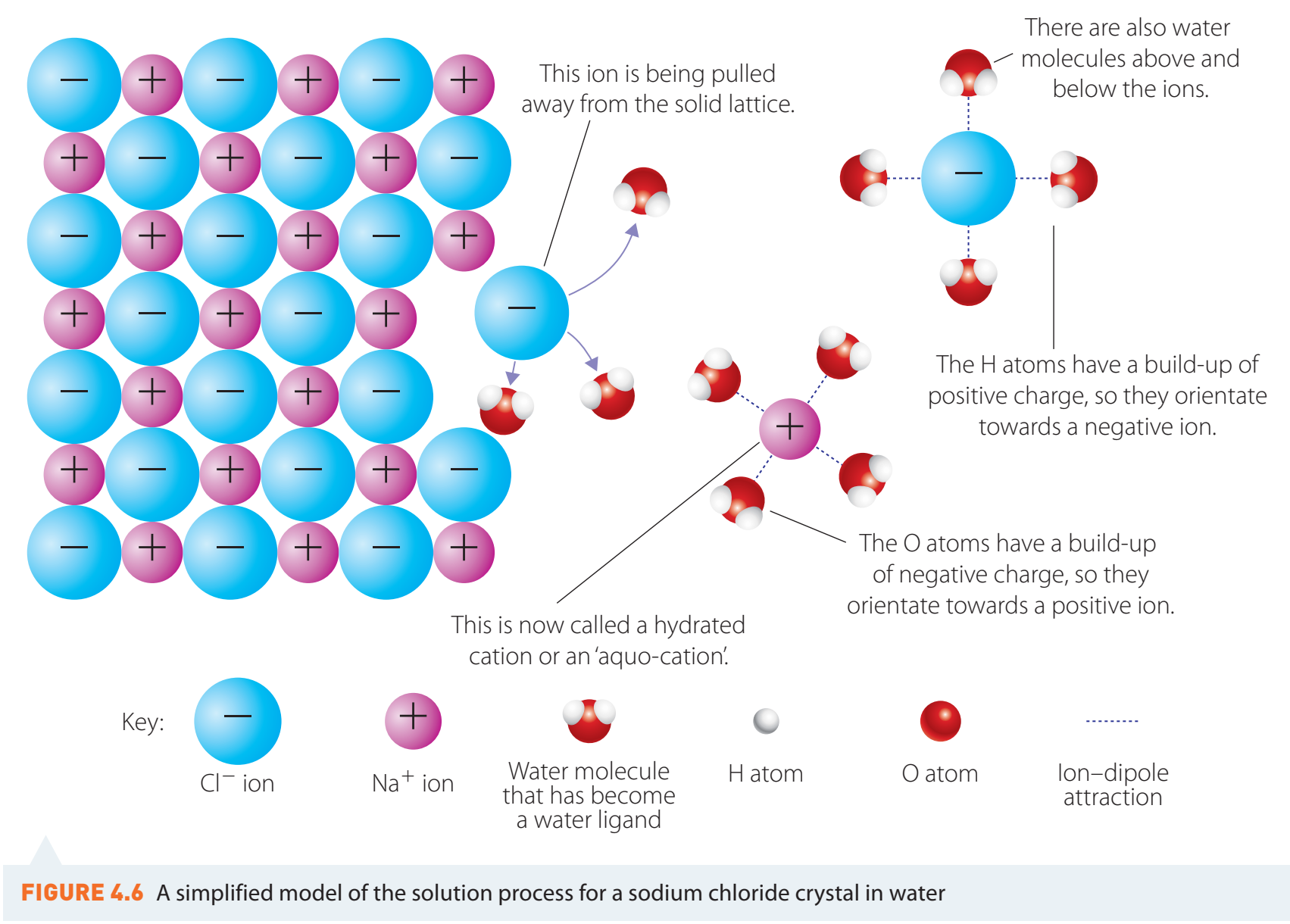
Dissolution is not disappearance. The substance maintains its chemical identity.
Physical Alterations
- Breaking: Exemplified by breaking a piece of chalk.
- Cutting: Such as slicing a block of cheese.
- Folding: Folding a piece of paper.
These actions alter shape or size without affecting chemical composition.
Addressing Misconceptions
Not all physical changes are reversible. For instance, grinding can permanently alter a substance's form, challenging reversibility.
Distinguishing Physical from Chemical Changes: Chemical changes result in new substances, while physical changes retain the original substance's identity.
Reversibility of Physical Changes
Reversibility: Changes that can be undone, restoring substances to their original state, are significant in everyday phenomena like ice melting and recycling.
- Phase changes: Including examples like water freezing and melting.
- Dissolution processes: Such as sugar dissolving in water.
Examples of Reversible Physical Changes
Water Freezing and Melting
- Ambient heat influences melting, while heat removal aids freezing.

Dissolution and Crystallisation of Sugar
- Sugar undergoes dissolution and crystallisation based on heat application and removal.
Sublimation and Deposition (Iodine)
- Demonstrates phase transition without passing through a liquid state.
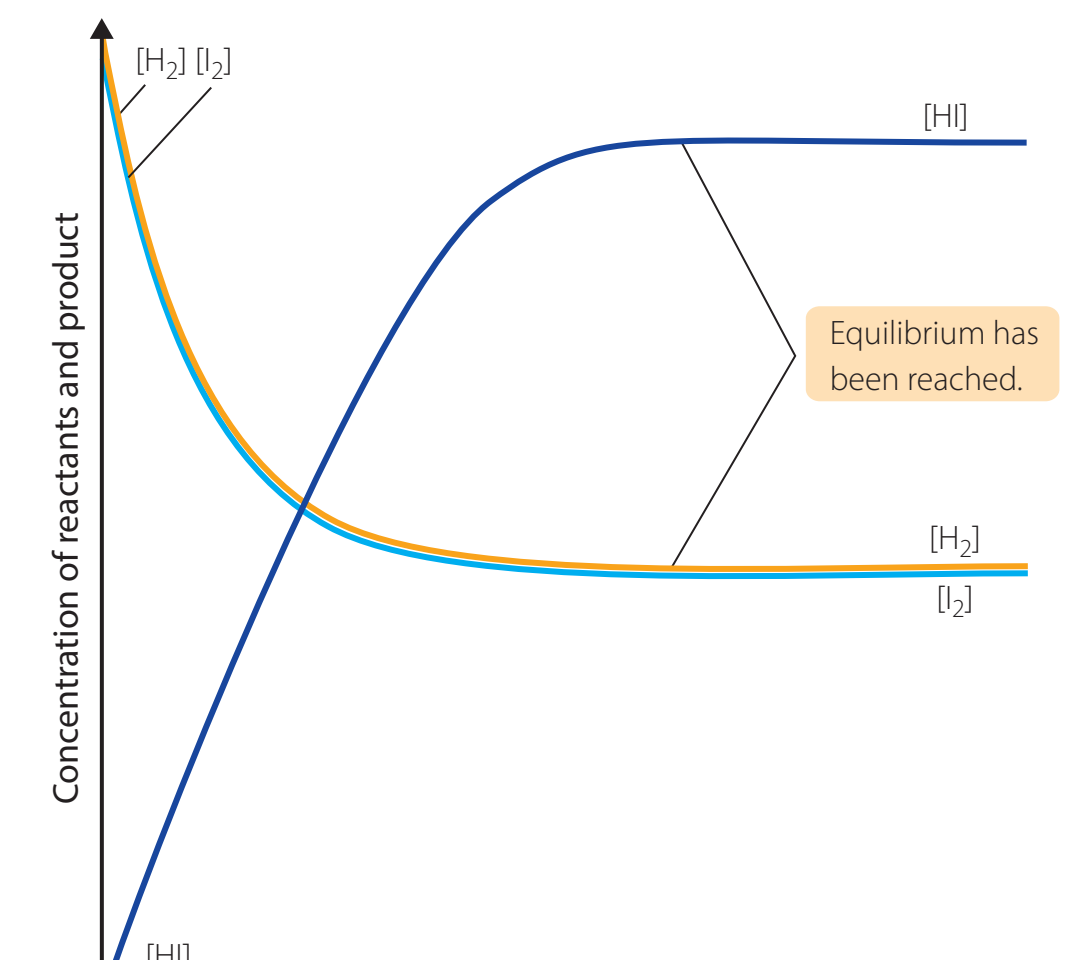
Role of Energy in Reversibility
- Endothermic processes: Require energy input, such as melting.
- Exothermic processes: Release energy, such as freezing.
Retention of Chemical Identity During Physical Changes
Chemical Identity: Represents a substance's chemical composition and structure, which remain unchanged during physical transformations.
- Chemical Identity: The chemical composition and structure that do not change during physical changes.
Contrast with Chemical Changes
- Chemical changes involve breaking and reforming bonds, resulting in new substances.
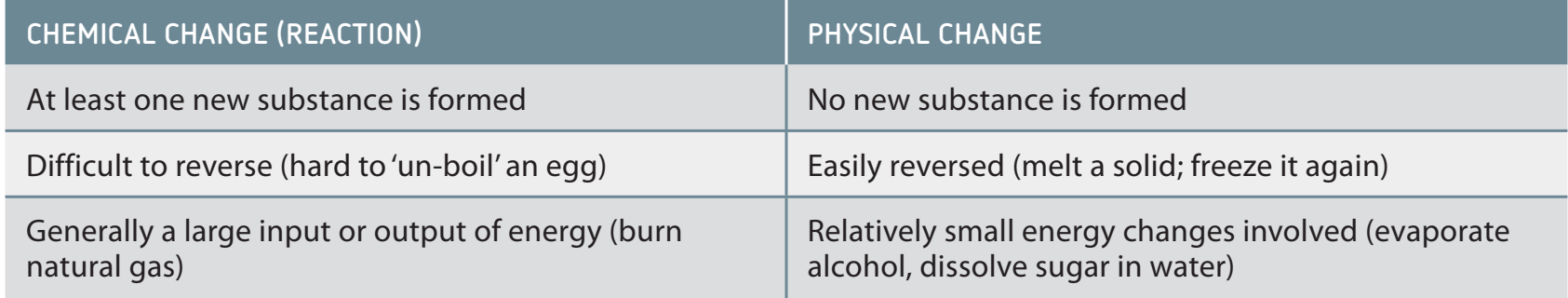
- Chemical changes result in new substances with different chemical identities.
500K+ Students Use These Powerful Tools to Master Physical Changes For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
442 flashcards
Flashcards on Physical Changes
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards40 quizzes
Quizzes on Physical Changes
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes7 questions
Exam questions on Physical Changes
Boost your confidence with real exam questions.
Try Chemistry Questions2 exams created
Exam Builder on Physical Changes
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Physical Changes
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Physical Changes you should explore
Discover More Revision Notes Related to Physical Changes to Deepen Your Understanding and Improve Your Mastery
