Photo AI
Last Updated Sep 24, 2025
Chemical Equilibrium Concepts Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Chemical Equilibrium Concepts quickly and effectively.
402+ students studying
Chemical Equilibrium Concepts
Overview of Chemical Equilibrium in Homogeneous Reactions
-
Dynamic Equilibrium: Dynamic Equilibrium is a condition in which the forward and reverse reaction rates are identical, leading to steady concentrations despite continuous reactions.
infoNoteDynamic Nature: While concentrations remain unchanged, equilibrium is dynamic because reactions occur continuously.
-
Significance: A firm grasp of equilibrium is crucial for anticipating reaction behaviours and in the development of industrial processes such as those in pharmaceuticals and materials synthesis.
Establishing Equilibrium
-
Conditions for Equilibrium:
- Closed System: No exchange of matter with the external environment.
- Stable Temperature: Required to maintain an equilibrium state.
-
Energy and Reaction Kinetics:
- Activation Energy: The minimum energy necessary for a reaction to proceed.
- Transition State Theory: Provides insight into how reactants form high-energy transition states before transforming into products.
Key Terms Definitions
-
Homogeneous Reaction: A reaction in which all reactants and products exist in the same phase.
-
Dynamic Equilibrium: Elucidates how molecular dynamics help maintain stable concentrations through ongoing reactions.
-
Equilibrium Constant (): A dimensionless value that is key to predicting the direction and extent of reactions.
chatImportantEquilibrium Constant (): Crucial for forecasting reaction outcomes.
Example Calculation:
- Consider the reaction :
- Compute the numerator: = .
- Compute the denominator: = .
- Calculate : .
- Consider the reaction :
Application of Homogeneous Equilibria in Real-World Processes
- Haber-Bosch Process: Applies equilibrium principles to efficiently synthesise ammonia by managing factors such as pressure and catalyst.
- Diverse Applications: Equilibrium principles are also utilised in various processes to optimise reaction conditions and enhance yields.
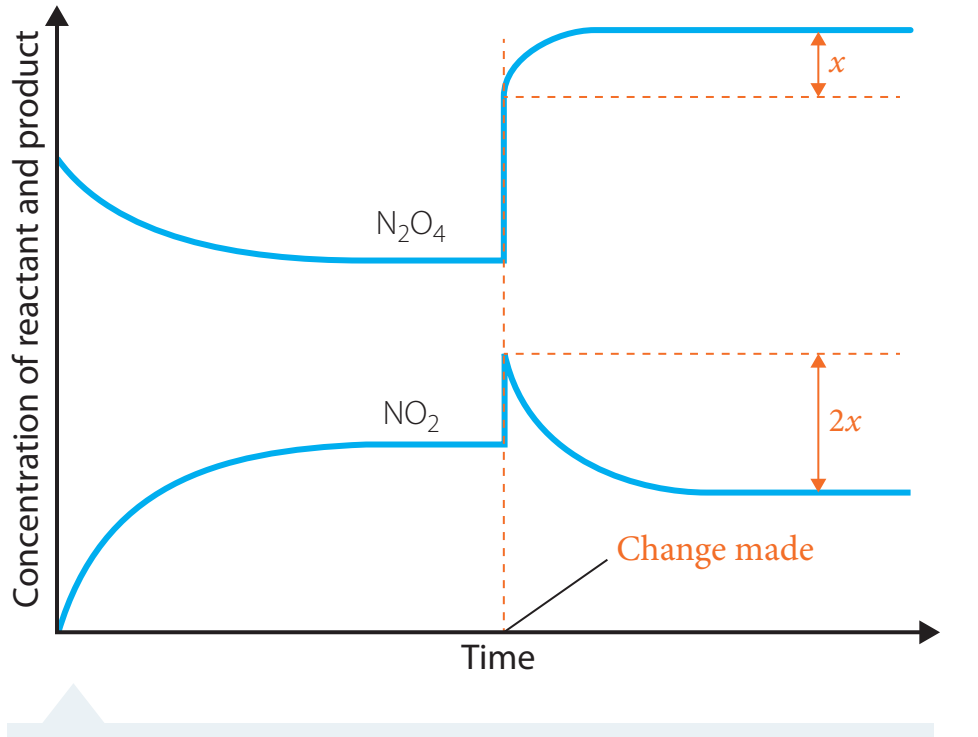
Deriving the Equilibrium Expression
Equilibrium Expression
- Definition: The ratio of product concentrations to reactant concentrations at equilibrium for a reversible reaction.
- Importance: Integral for predicting chemical behaviour by indicating concentration balance tendencies.
Derivation Process
Step-by-Step Guide:
- 1. Start with a Balanced Chemical Equation
- Ensure reactants and products have balanced atoms.
- 2. Formulate the Equilibrium Expression
- Use concentrations: [A], [B] for reactants; [C], [D] for products.
- 3. Apply Stoichiometric Coefficients
- Include coefficients as exponents:
- Include coefficients as exponents:
Worked Examples
Example 1: Synthesis of Ammonia
- Balanced Equation:
- Derivation of :
Common Misconceptions
- Exclude solids or pure liquids from expressions. Only gaseous and dissolved species are included.
- Distinguish between Q and : Q is applicable at any stage of the reaction, while is specific to equilibrium.

Calculating the Equilibrium Constant
Steps for Calculation
-
Step 1: Write the balanced chemical equation.
chatImportantCorrect balancing is fundamental. Errors in balancing impact accuracy.
-
Step 2: Arrange initial and equilibrium concentration data in a table format for clarity.
-
Step 3: Formulate and resolve the equilibrium expression.
Be cautious of common pitfalls such as unit inconsistencies in calculations.
Example Problems
- Example 1: Reaction
- Determine using initial concentration data.
Predicting Reaction Directions Using
Magnitude of
- : Indicates product predominance.
- : Indicates reactant predominance.
- : A balanced state, with neither reactants nor products favoured.
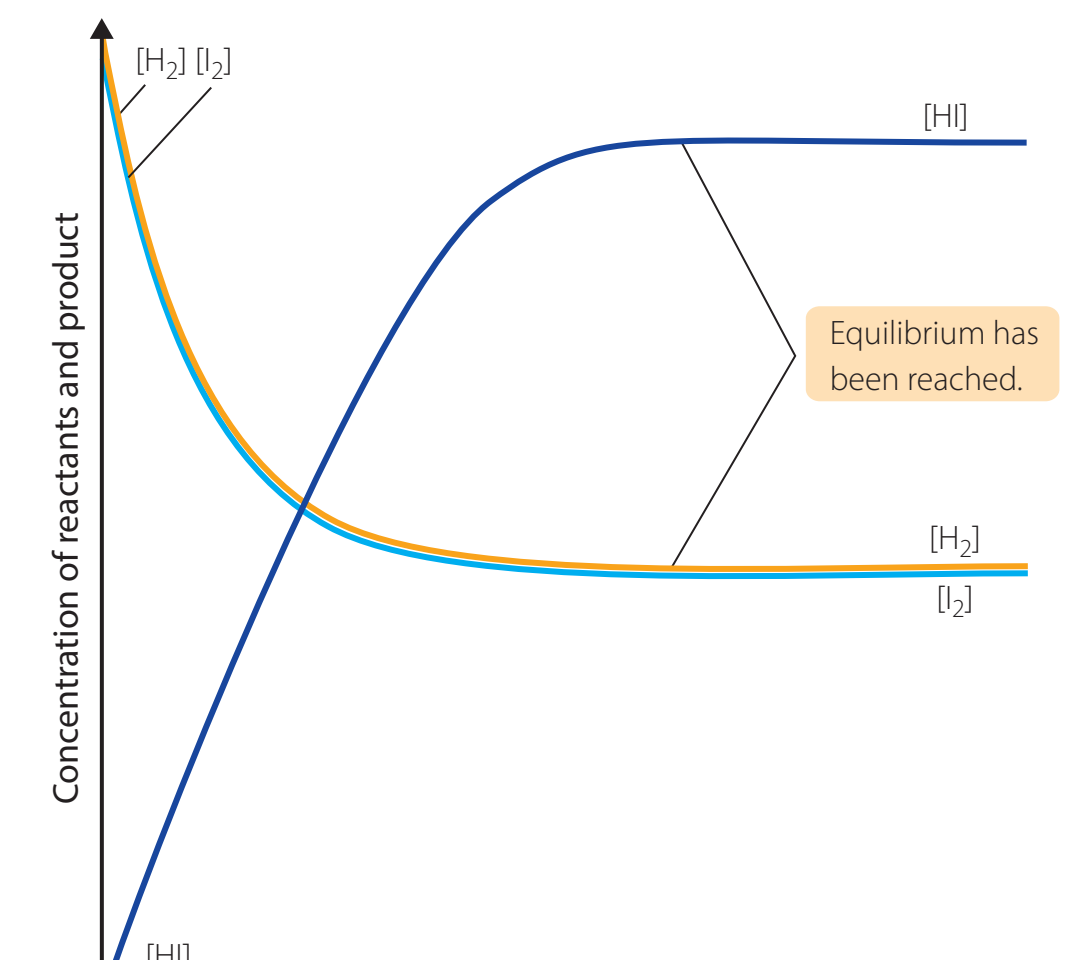
Using Reaction Quotient (Q)
-
Reaction Quotient (Q): The ratio of products to reactants at any point, utilised to assess a system's proximity to equilibrium.
chatImportantComparing Q with is vital for predicting directional shifts.
Examples and Exercises
Example 1: Industrial ammonia synthesis using the Haber process.
Exercise: Calculate Q for specified initial conditions to discern reaction directionality.
- Solution: For the reaction with initial concentrations , and , calculate: Since (assuming at standard conditions), the reaction will proceed toward products.
Effect of Conditions on
Concentration and Pressure
- Modifications in concentration and pressure do not influence .
Clarification: While concentration and pressure affect equilibrium position, they do not alter .
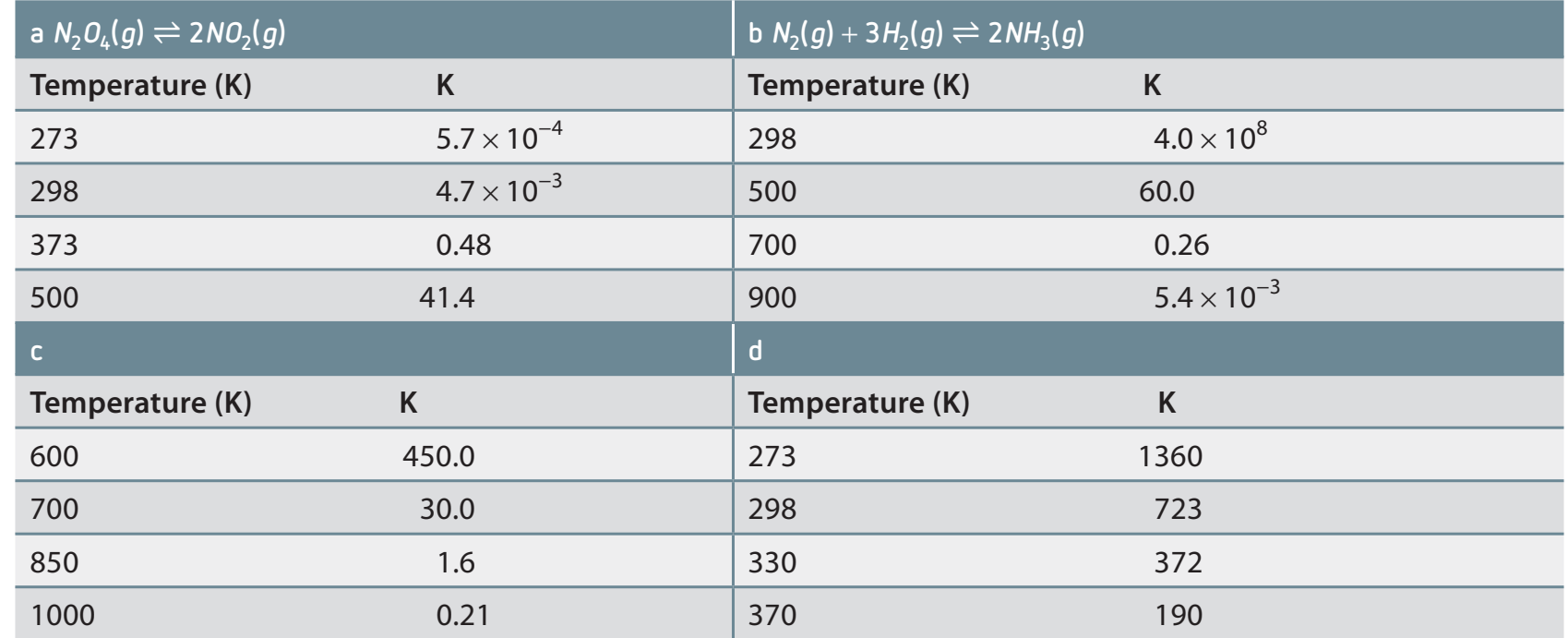
Temperature Effect
-
Temperature Dependence: Directly affects due to changes in kinetic energy.
-
Examples:
- Exothermic Reactions: Elevated temperature reduces .
- Endothermic Reactions: Elevated temperature increases .
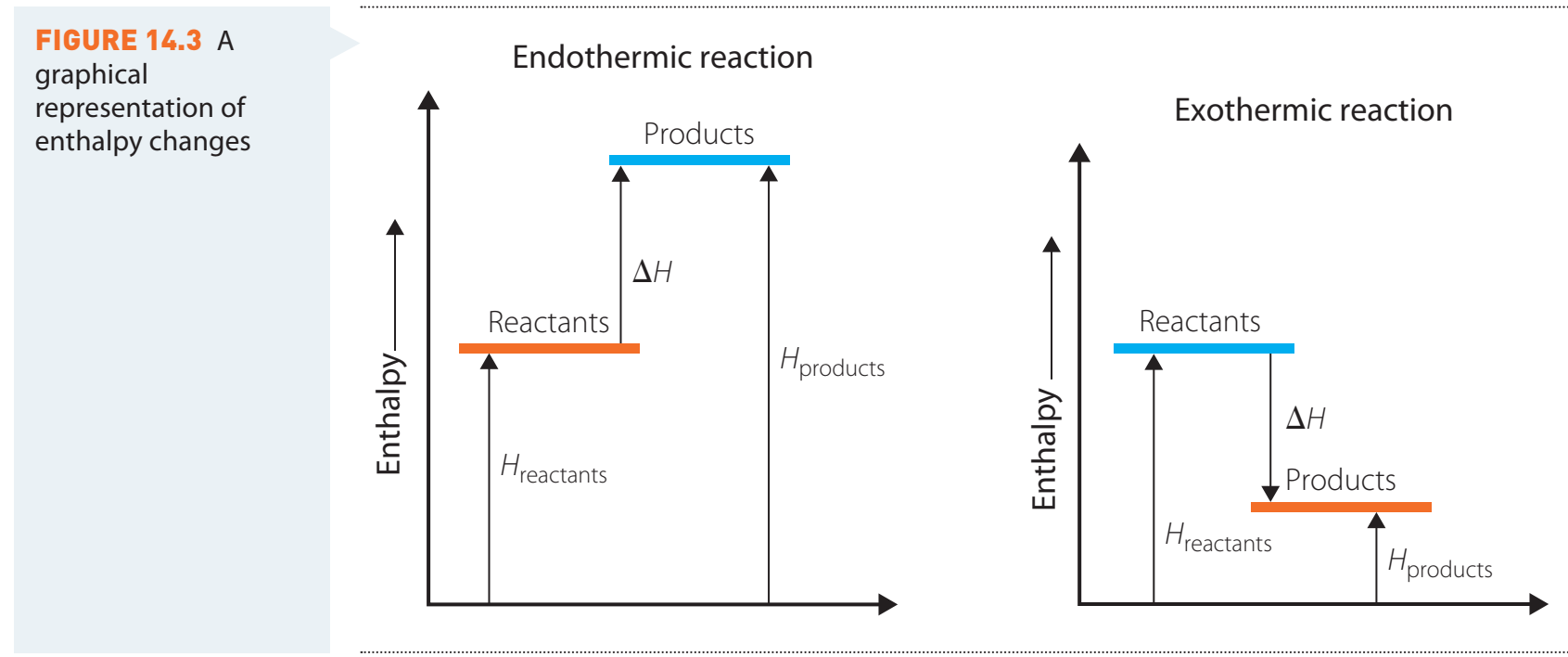
Effect on vs
- vs
- is pressure-variable.
- is pressure-independent but varies with temperature.
Introduction to ICE Tables
ICE Tables: Structured tools designed for tracking concentration changes in reaction species.
Setting Up ICE Tables
- Initial, Change, Equilibrium: Record these distinct stages in a tabular layout.
| Species | Initial | Change | Equilibrium |
|---|---|---|---|
| Reactant A | X mol/L | -Y mol/L | (X-Y) mol/L |
| Reactant B | Z mol/L | -Y mol/L | (Z-Y) mol/L |
| Product C | 0 mol/L | +Y mol/L | Y mol/L |
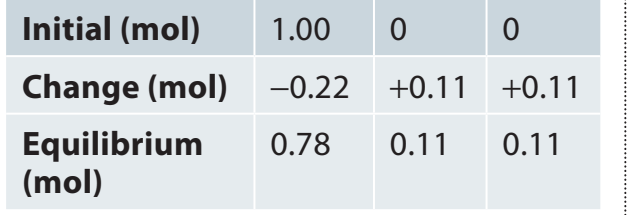
Worked Example
-
Example: For the reaction with initial concentrations , and , if , find the equilibrium concentrations.
Solution:
-
Set up ICE table:
Species Initial Change Equilibrium A 0.5M -x (0.5-x)M B 0.5M -x (0.5-x)M C 0M +x xM -
Apply equilibrium constant expression:
-
Solve for x:
-
Using the quadratic formula: Since we need a real solution and cannot be negative,
-
Equilibrium concentrations:
-
Common Mistakes and Misconceptions
- Balancing Equations: Errors frequently arise from improper balancing.
Consistency minimises errors: Regularly verify calculations against accurate references.
-
Ignoring Stoichiometry: Incorrect application of stoichiometry results in inaccuracies.
-
Ignoring Phases: Excluding phases such as solids or liquids from equilibrium expressions is inaccurate.
Corrective Strategies
- Use of Q: Gain insight into real-time adjustments by utilising Q prior to equilibrium.
- Checking Computations: Employ dimensional analysis to verify accuracy.
500K+ Students Use These Powerful Tools to Master Chemical Equilibrium Concepts For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
170 flashcards
Flashcards on Chemical Equilibrium Concepts
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards15 quizzes
Quizzes on Chemical Equilibrium Concepts
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes5 questions
Exam questions on Chemical Equilibrium Concepts
Boost your confidence with real exam questions.
Try Chemistry Questions2 exams created
Exam Builder on Chemical Equilibrium Concepts
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Chemical Equilibrium Concepts
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Chemical Equilibrium Concepts you should explore
Discover More Revision Notes Related to Chemical Equilibrium Concepts to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Calculating the Equilibrium Constant
Calculating Equilibrium Constants
257+ studying
197KViews96%
114 rated
Calculating the Equilibrium Constant
Equilibrium Constant Applications
263+ studying
199KViews96%
114 rated
Calculating the Equilibrium Constant
Equilibrium Constant Calculations
426+ studying
181KViews