Photo AI
Last Updated Sep 24, 2025
Equilibrium Constant Calculations Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Equilibrium Constant Calculations quickly and effectively.
281+ students studying
Equilibrium Constant Calculations
Introduction
Understanding the importance of calculations in equilibrium analysis is crucial. Errors can lead to:
- Misinterpretation of equilibrium states.
- Faulty predictions about reaction behaviours.
Definition and Significance
- Equilibrium Constant (): The ratio of concentrations of products to reactants at equilibrium, each raised to their stoichiometric coefficients.
- Equilibrium Constant (): Demonstrates the balance within a chemical reaction at equilibrium by comparing products to reactants.
- Significance: Illustrates how far a reaction proceeds to completion, indicating relative amounts of reactants and products.
Understanding the Equilibrium Constant ()
The equilibrium constant is crucial in chemistry, providing insight into the extent of chemical reactions. Calculations ensure accurate predictions about product and reactant concentrations at equilibrium.
Steps for Calculating
Setup of Balanced Chemical Equations
- Key Point: Balancing equations is essential to accurate calculations. It ensures the proper stoichiometric ratios are maintained.
Common Pitfalls in Balancing Equations
- Ensure coefficients accurately reflect stoichiometry.
- Maintain correct order and proportion of elements.
- Examples:
- Simple Reaction:
- Complex Reaction:
Initial and Equilibrium Concentrations
- Role: Initial concentrations are foundational for determining equilibrium states.
- Example Setup: Use tables for transparency in data representation.
- Initial: ,
- Transition: Calculate change () to reach equilibrium.
Table Representation of Concentrations
| State | [A] | [B] |
|---|---|---|
| Initial | ||
| Change | ||
| Equilibrium |
Use of the Equilibrium Expression
- Formulation Process: Based on the balanced equation, using stoichiometric coefficients.
- Example Expression:
- For ,
- Unit Consistency: Ensure all terms in the expression are dimensionless or have consistent units.
Example Calculations
Homogeneous Equilibria
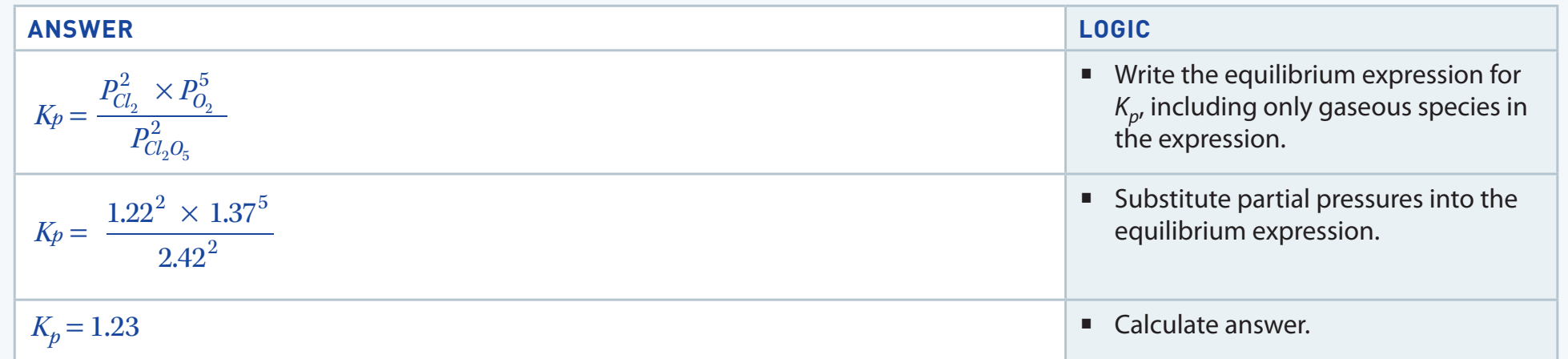
- Problem: Calculate for
- Solution:
- Write the equation:
- Record initial values: Let's say , ,
- At equilibrium: , ,
- Apply the equilibrium expression:
Heterogeneous Equilibria
- Differences: Phase exclusions are important, focusing only on gases and aqueous solutions.
- Example: yields
- Since solids have constant activities, they are excluded from the equilibrium expression.
- If at equilibrium, then
Significant Figures and Units Considerations
- Importance: Use significant figures for precision in .
Common Mistakes in Handling Units
- Conversion Factors: Do not ignore them.
- Unit Misuse: Ensure consistent unit use across calculations.
Introduction to ICE Tables
ICE Tables: Initial, Change, and Equilibrium tables offer a structured way to monitor reactant and product concentrations, simplifying equilibrium calculations.
- Purpose: Aid in organising and simplifying complex reaction data.
ICE Tables effectively organise data and simplify equilibrium calculations.
Structure and Components of ICE Tables
-
Initial Concentrations:
- Record starting concentrations.
- Products are typically zero initially.
-
Change in Concentrations:
- Use 'x' to represent shifts to equilibrium.
- Change direction (+/-) based on forward or reverse reaction.
-
Equilibrium Concentrations:
- Calculate by adjusting initial values using changes.
Detailed Examples
Example 1: Simple Homogeneous Reaction
- Reaction Setup:
- Step-by-Step Process:
- Initial Values: , , .
- Define Change: , .
- Calculate Equilibrium: If , then: Solving: Using the quadratic formula: Therefore: and
Example 2: Heterogeneous Reaction
- Reaction Context:
- Considerations:
- Exclude solids from equilibrium calculations, focus on .
- If initially and at equilibrium
- Then
Visual Representations and Diagrams
Common Pitfalls and Strategic Approaches
- Incorrect sign use in 'Change' row.
- Do not include solids or liquids in equilibrium constants.
- Strategic Tips:
- Validate work with logical checks.
- Ensure consistency in units.
Introduction to Reaction Quotient (Q)
- Reaction Quotient (Q): The Reaction Quotient is the ratio of the concentrations of products to reactants, each raised to the power of their coefficients. It determines how a reaction will proceed to reach equilibrium.
To calculate , use the formula:
- Products: Concentrations of species on the right side of the reaction.
- Reactants: Concentrations of species on the left side of the reaction.
- Comparison with :
- represents the reaction's condition at equilibrium.
- Q can be calculated at any point in a reaction, while is a measure solely at equilibrium.
Remember: is only accurate when the reaction is at equilibrium, whereas Q helps predict the direction to get there.
Predicting Reaction Progression Using Q versus
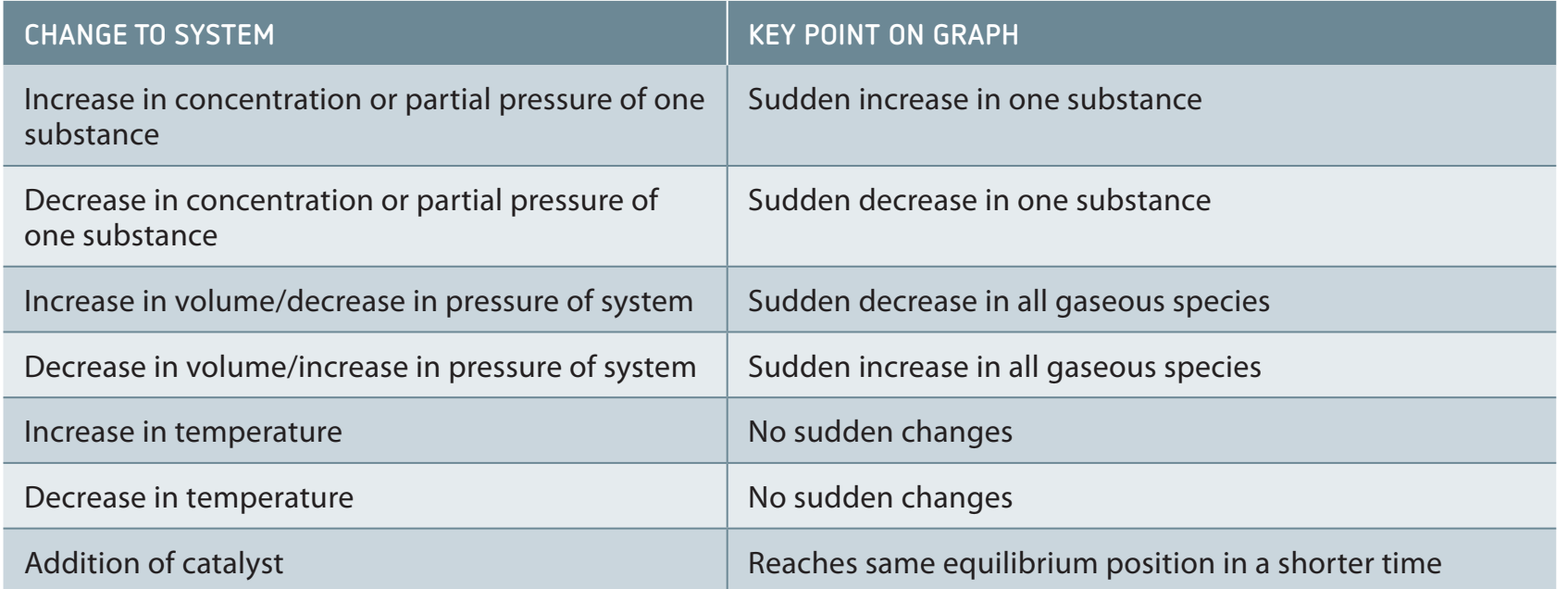
- Conceptual Comparison:
- The relationship between Q and dictates how a reaction progresses towards equilibrium.
Understanding the Scenarios
-
Q < :
- Reaction Progress: Moves towards products. Indicates a forward shift.
infoNoteWhen Q is less than , the reaction proceeds forward to increase product concentration.
-
Q = :
- System Equilibrium: No net change.
infoNoteThe reaction is at equilibrium when Q equals . No further shift.
-
Q > :
- Reaction Progress: Moves towards reactants. Indicates a reverse shift.
infoNoteWhen Q is greater than , the reaction will shift backward to increase reactant concentration.

Worked Examples
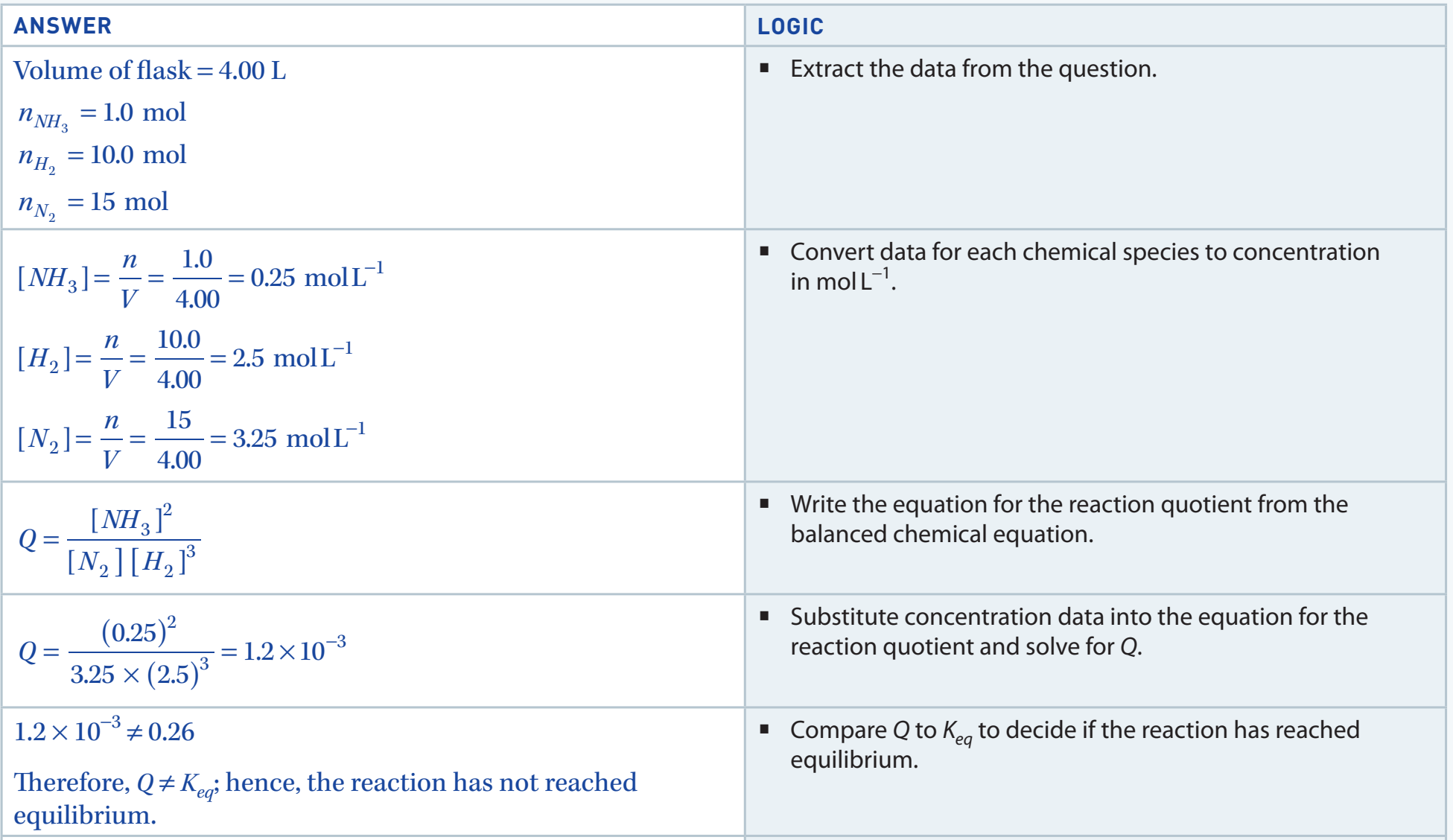
Example 1: Q <
- Step-by-Step Calculation:
- Consider the reaction:
- Given: , , ,
- Calculate :
- Since , the reaction will proceed in the forward direction (towards products).
Outcome: Reaction will shift towards the products.
Example 2: Q >
- Step-by-Step Calculation:
- Consider the reaction:
- Given: , , ,
- Calculate :
- Since , the reaction will proceed in the reverse direction (towards reactants).
Outcome: Reaction will shift towards the reactants.
Common Mistakes:
Incorrect Use of Units
- Common Conversion Errors:
- Moles to litres
- Grams to moles
- Atmospheres instead of pascals
- Conversion Tip:
- Ensure consistency: Units must be consistent across all calculations.
Stoichiometric Coefficients Mistakes
- Errors: Occur when stoichiometric coefficients are improperly applied.
- Example Scenario: Using incorrect coefficients, e.g.,
- Check: Ensure stoichiometry after balancing chemical equations.
Inaccurate Equilibrium Expressions
- Correction Steps:
- Initial Check: Verify inclusion of all products and reactants.
- Validation Step:
- Confirm exponents match stoichiometric coefficients.
- Final Step:
- Clarify omitted species with specific examples.
Challenges in Calculating
Troubleshooting Tips and Corrective Strategies
- Key Steps:
- Verify: Balance of chemical equations.
- Units Check: Ensure unit consistency.
- Correct: Stoichiometric errors thoroughly.
- Rewrite: Ensure equilibrium expressions are accurate.
Addressing Misconceptions and Their Implications
- Common Misconceptions:
- "Equilibrium means equal concentrations": Incorrect perception.

500K+ Students Use These Powerful Tools to Master Equilibrium Constant Calculations For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
170 flashcards
Flashcards on Equilibrium Constant Calculations
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards15 quizzes
Quizzes on Equilibrium Constant Calculations
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes5 questions
Exam questions on Equilibrium Constant Calculations
Boost your confidence with real exam questions.
Try Chemistry Questions2 exams created
Exam Builder on Equilibrium Constant Calculations
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Equilibrium Constant Calculations
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Equilibrium Constant Calculations you should explore
Discover More Revision Notes Related to Equilibrium Constant Calculations to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Calculating the Equilibrium Constant
Calculating Equilibrium Constants
342+ studying
180KViews96%
114 rated
Calculating the Equilibrium Constant
Equilibrium Constant Applications
476+ studying
183KViews96%
114 rated
Calculating the Equilibrium Constant
Chemical Equilibrium Concepts
464+ studying
184KViews
