Photo AI
Last Updated Sep 24, 2025
Chemical Reactions Quantitative Calculations Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Chemical Reactions Quantitative Calculations quickly and effectively.
304+ students studying
Chemical Reactions Quantitative Calculations
Introduction to Key Concepts
- Law of Definite Proportions: A chemical compound consistently contains elements in the same mass proportion.
- Example: In the synthesis of ammonia for fertilisers, the mass ratio of nitrogen to hydrogen remains constant.
Mole Ratio: Refers to the ratio between the amounts in moles of any two compounds involved in a chemical reaction, crucial in sectors such as pharmaceuticals.
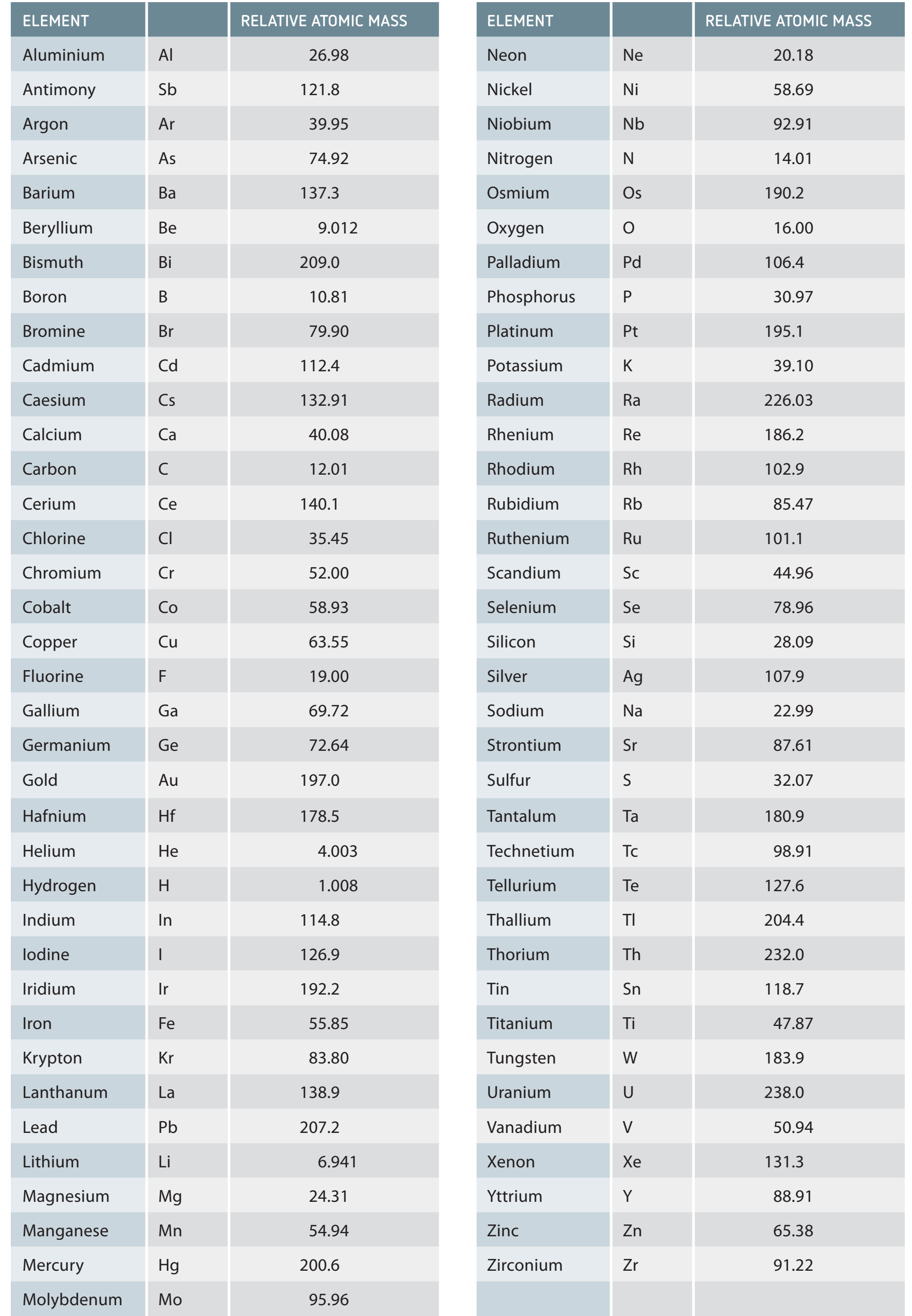
Concept of Molar Mass
- Molar Mass: The mass of one mole of a substance, expressed in grams per mole (g/mol).
- Facilitates conversion between atomic and large-scale measurements using Avogadro's Number: particles/mole.
Molar Mass: The mass of one mole of a substance, expressed in grams per mole (g/mol).
Examples:
- Hydrogen:
- Atomic Mass: 1.01 g/mol
- Sodium Chloride (NaCl):
- Sodium (Na): 22.99 g/mol
- Chlorine (Cl): 35.45 g/mol
- Total Molar Mass: 58.44 g/mol
Practical Investigations
- Objective: Conduct experiments to determine molar mass.
- Procedure:
- Weigh the piece of magnesium.
- Heat it to allow reaction with oxygen.
- Weigh Again to assess the change in mass.
Understanding the Mole and Avogadro's Constant
- Definition of the Mole: The mole is a standard unit for quantifying atoms or molecules.
- Avogadro's Contribution (1811): Established that gas volumes are proportional to the number of molecules.
- Avogadro's Constant: particles per mole.
Percent Composition and Empirical Formula
Percent Composition: Percentage by mass of each element within a compound.
-
Percent Composition Formula:
-
Empirical Formula: The simplest whole-number ratio of elements in a compound.

Visual Aids
- Empirical vs. Molecular Formulae:
- Example:
- Glucose Molecular Formula: C₆H₁₂O₆
- Empirical Formula: CH₂O
- Example:
Limiting Reagent
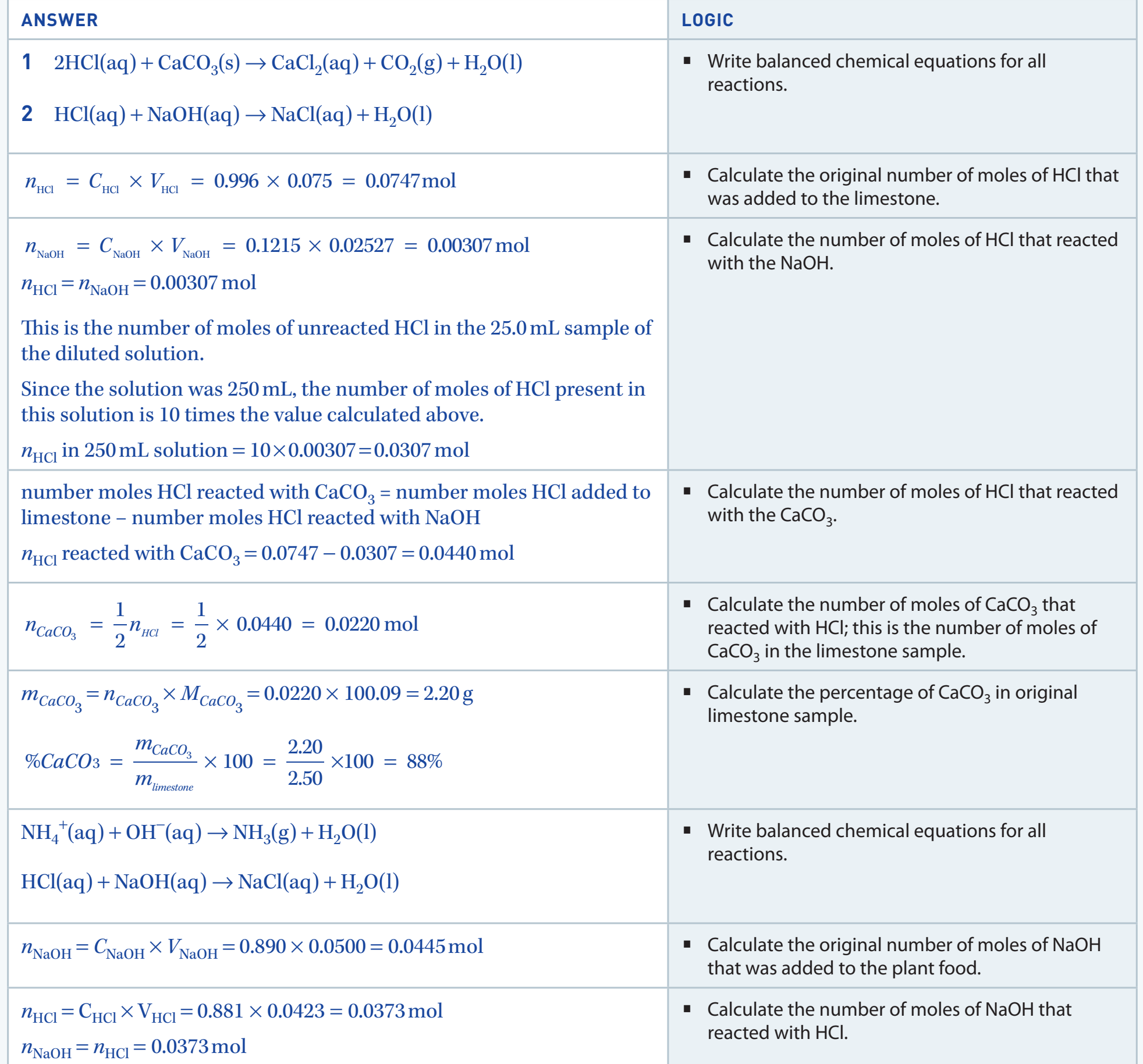
- Limiting Reagent: The reactant that is entirely consumed first, determining the amount of product formed.
Limiting Reagent: It is the reactant that is used up first and therefore limits the amount of product formed.
Example Analysis: Reaction of Hydrogen and Oxygen
- Balanced Equation:

Formulae and Calculation Techniques
- Molar Mass: Molar Mass =
- Number of Moles:
- Avogadro's Number:
- Percentage Composition:
Worked Example
Calculate the molar mass of glucose (C₆H₁₂O₆):
-
First, identify the atomic mass of each element:
- Carbon (C): 12.01 g/mol
- Hydrogen (H): 1.01 g/mol
- Oxygen (O): 16.00 g/mol
-
Multiply each atomic mass by the number of atoms:
- Carbon: 12.01 g/mol × 6 = 72.06 g/mol
- Hydrogen: 1.01 g/mol × 12 = 12.12 g/mol
- Oxygen: 16.00 g/mol × 6 = 96.00 g/mol
-
Add all contributions to find the total molar mass:
- Total: 72.06 + 12.12 + 96.00 = 180.18 g/mol
Therefore, the molar mass of glucose is 180.18 g/mol.
500K+ Students Use These Powerful Tools to Master Chemical Reactions Quantitative Calculations For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
234 flashcards
Flashcards on Chemical Reactions Quantitative Calculations
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards27 quizzes
Quizzes on Chemical Reactions Quantitative Calculations
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes21 questions
Exam questions on Chemical Reactions Quantitative Calculations
Boost your confidence with real exam questions.
Try Chemistry Questions1 exams created
Exam Builder on Chemical Reactions Quantitative Calculations
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Chemical Reactions Quantitative Calculations
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Chemical Reactions Quantitative Calculations you should explore
Discover More Revision Notes Related to Chemical Reactions Quantitative Calculations to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Chemical Reactions and Stoichiometry
Law of Conservation of Mass
273+ studying
192KViews