Photo AI
Last Updated Sep 26, 2025
Law of Conservation of Mass Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Law of Conservation of Mass quickly and effectively.
346+ students studying
Law of Conservation of Mass
Introduction to the Law of Conservation of Mass
Antoine Lavoisier: Fundamental in establishing the Law of Conservation of Mass. His work confirmed that the total mass remains unchanged in a closed system.
-
Notable Experiments:
- Combustion of Phosphorus and Sulphur:
- Demonstrated mass consistency when reactions occurred in sealed environments.
- Utilised quantitative methods to confirm all matter was conserved.
- Mercury Oxide Experiment:
- Heated mercury oxide to reveal conservation of oxygen and mercury in a closed flask.
- Illustrated that reactions involve atom rearrangement, not mass alteration.
- Combustion of Phosphorus and Sulphur:
-
18th Century Scientific Context:
- An era characterised by growing empirical evidence refuting outdated theories.
- Joseph Priestley, a leading proponent of phlogiston theory, observed its disproof through Lavoisier's focus on oxygen.
Lavoisier's Innovations: Proved mass constancy and set the stage for modern chemical analysis with empirical rigour.
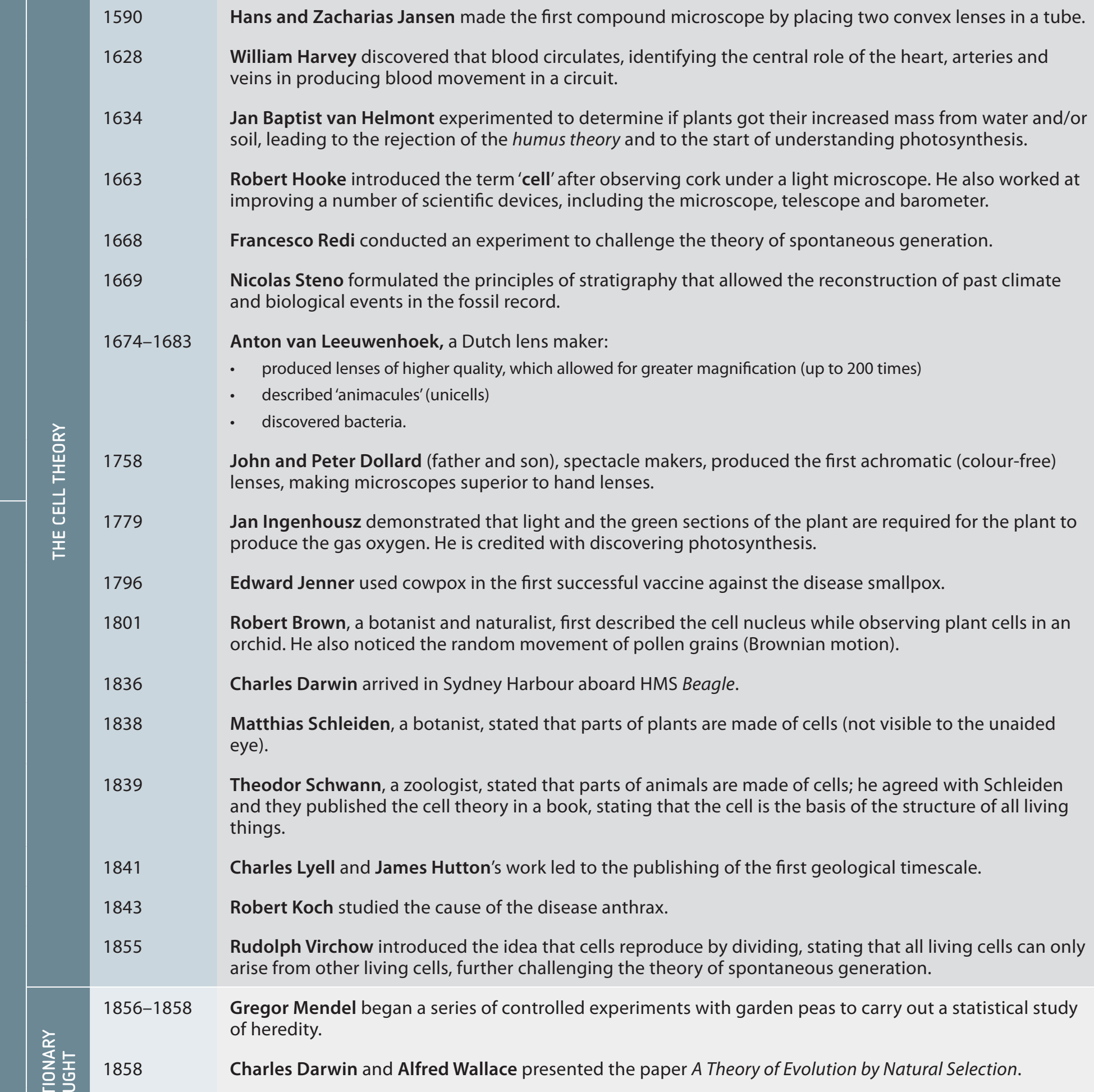
Introduction to Balancing Equations
Chemical Equation: A symbolic depiction of a chemical reaction, with reactants on the left and products on the right.
-
Reactants: Substances existing at the start of a reaction. Example: Hydrogen and oxygen that form water.
-
Products: Substances generated by a reaction. Example: Water formed from hydrogen and oxygen.
-
Balancing is essential due to the Law of Conservation of Mass:
- Mass is neither created nor destroyed.
- Ensures equality in the number of atoms of each element on both sides.
Fundamental Principles
- Conservation of Atoms: List each atom type distinctly to ensure equilibrium.
- Conservation of Mass: Total reactant mass equals total product mass.
- Subscripts remain unchanged: They define compound identity.
Coefficients: Can be altered to balance equations.
- Example: Adjust the coefficients of water (H₂O) and hydrogen (H₂).
Step-by-Step Approach
Conversion and Balancing Method
- Translate word equations into chemical formulas.
- Write the unbalanced equation.
- Tally atoms for each element among reactants and products.
- Initiate with atoms appearing in the fewest compounds.
- Balance carbon and hydrogen initially, followed by oxygen.
- Vary coefficients to align the atoms.
- Recount atoms for verification.
- Simplify coefficients to their minimal ratios.
Tip: Initiate with the most complex molecule for easier balancing.
Example Walkthroughs
Methane Combustion
- Equation: CH₄ + O₂ → CO₂ + H₂O
- Balancing Steps:
- Translate the equation to include chemical formulas.
- Balance carbon (C), hydrogen (H), then oxygen (O). Results in: CH₄ + 2O₂ → CO₂ + 2H₂O.
Phosphorus Trichloride Synthesis
- Equation: P₄ + Cl₂ → PCl₃
- Balancing Steps:
- Balance phosphorus (P) initially.
- Then balance chlorine (Cl).
- Results in: P₄ + 6Cl₂ → 4PCl₃
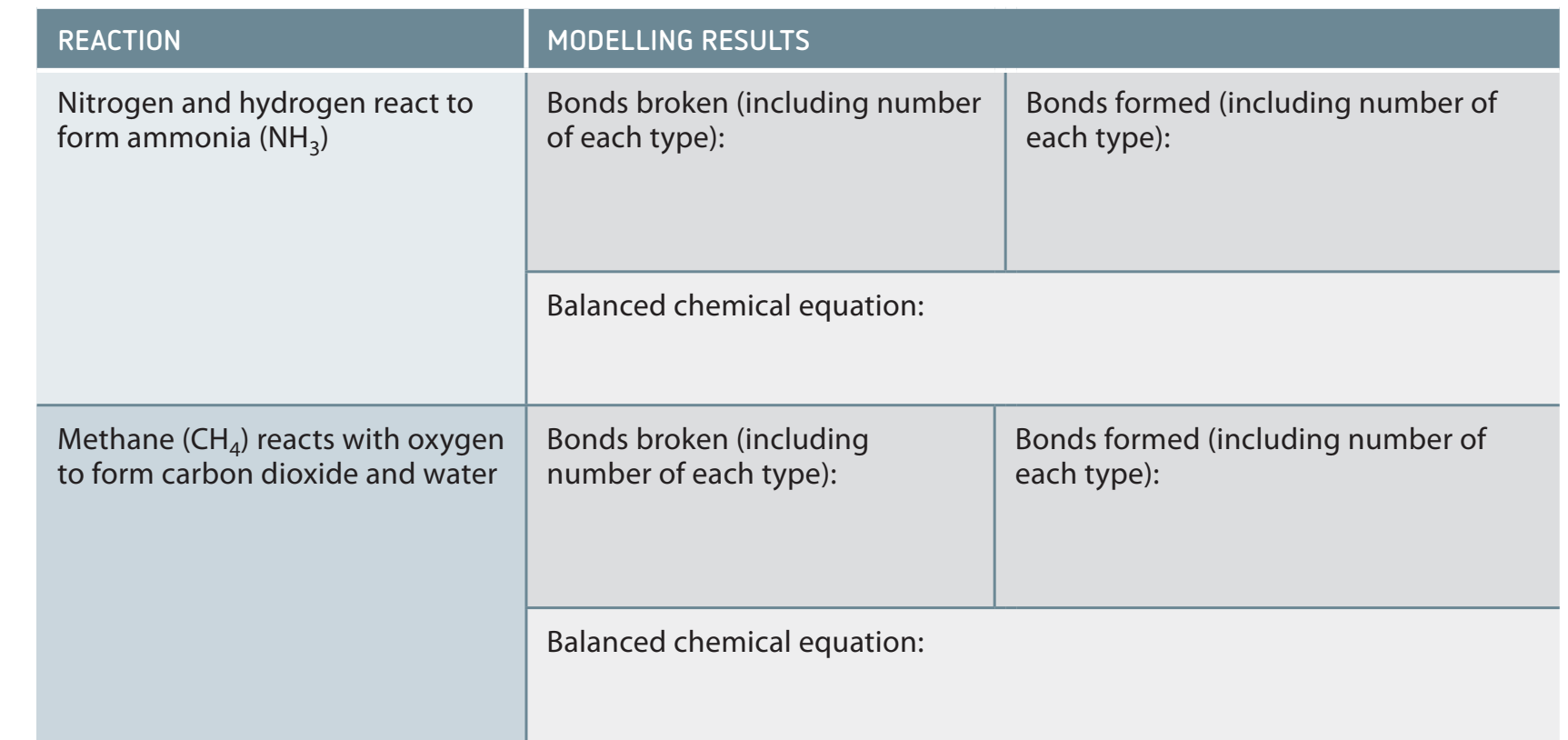
Introduction to Stoichiometry and Mass Conservation
- Stoichiometry: Quantitative connections between reactants and products in chemical reactions.
- Based on the Law of Conservation of Mass, where reactant mass equals product mass.
From Equations to Anticipations: Balanced equations enable the prediction of mass variations in reactions.
Importance of Balanced Equations
- Balanced Equations: Preserve mass and atom conservation in a reaction.
- Analogy: Comparable to a recipe ensuring proper ingredient amounts for desired outcomes.
- Essential in industries for scaling reactions and obtaining predictable results.
Worked Example: Oxygen Mass Calculation in Hydrogen Combustion
Question: How much oxygen is necessary to completely combust 10 g of hydrogen?
-
Balanced Equation:
-
Given: 10 g of .
-
Solution Steps:
- Convert mass of to moles: .
- From equation: 2 moles of react with 1 mole of .
- Calculate moles of : .
- Convert to mass of : .
Overview of Mass Changes
- Key Concepts:
- Law of Conservation of Mass: Total mass remains stable during chemical reactions.
- Stoichiometry: Used to foresee, comprehend, and compute mass changes in reactions.
Law of Conservation of Mass: Total mass remains unchanged during chemical reactions.
Mass Changes through Representative Stoichiometry Problems
- Problem Type: Determine mass balance by assessing initial and final amounts.
- Approach Steps:
- Identify known and unknown amounts.
- Compose and balance the chemical equation.
- Utilise molar ratios and stoichiometric coefficients:
- Relate reactants to products.
- Perform mass computations to verify mass balance.
Question: Why might mass seem to change in an open system?
Problem Examples
Worked Example: Magnesium Oxide Production
- Steps for Calculation:
- Known Quantities: Identify the mass of magnesium and oxygen.
- Balanced Equation:
- Reaction:
- Mass Calculation:
- Convert mass to moles and verify mass conservation from reactants to products.
- Outcome Example:
- Calculation: 48 g of Mg reacts with 32 g of O₂ to produce 80 g of MgO, confirming mass conservation.
Practical Implications
- Systems Examination:
- Closed Systems: Accurately conserve mass.
- Open Systems: May display apparent mass losses due to factors like gas escape.
- Real-world Examples:
- Industrial applications utilise mass conservation for efficiency.
- Environmental monitoring employs mass balance to track pollutants.
Tips and Tricks for Chemistry Exams
Strategies for Avoiding Mistakes
Balancing Equations
-
Common Errors:
- Miscounting Atoms: Overlooking complex compounds results in incorrect atom counts.
- Neglecting Verification: Always verify atom counts after balancing.
-
Balancing Strategy Checklist:
- Identify Elements: Note all elements involved in the reaction.
- Initial Tally: Count each atom type on both sides.
- Modify Coefficients: Adjust coefficients to balance the atoms.
- Reassess Counts: Verify all elements for balance.
Worked Example:
- Example for balancing equation :
- Balance Carbon: Start with carbon atoms.
- Balance Hydrogen: Follow with hydrogen atoms.
- Adjust Oxygen: Increase oxygen to match others.
- Final Balanced Equation:
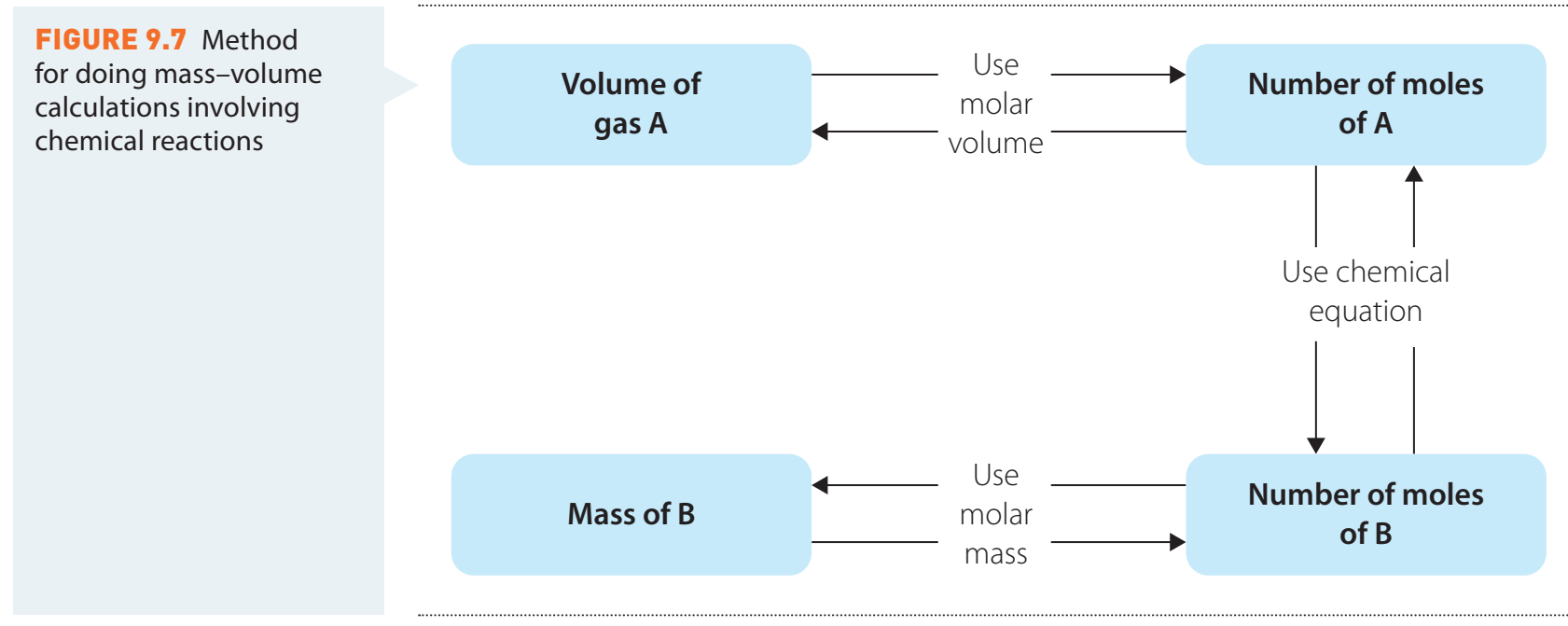
Importance of Units and Significant Figures
Quick Guide to Units
| Conversion | Multiplier |
|---|---|
| Grams to Moles | |
| Moles to Particles |
Significant Figures:
- Significance:
- Crucial for precision. Incorrect application can significantly alter results.
- Strict adherence to guidelines is necessary for accuracy.
Work Organisation and Exam Techniques
Organisational Strategies
-
Time Management Checklist:
- Allocate time for reading, solving, and reviewing wisely.
- Modify based on question difficulty and familiarity.
-
Problem Approach Flowchart:
- Read Instructions Clearly: Fully comprehend question requirements.
- Plan Approach: Choose suitable formulas and data.
- Execute Solution: Conduct computations carefully.
- Review: Scrutinise results for possible inaccuracies.
Definitions Recap
- Molar Mass: Mass of one mole of a substance, typically in grams per mole.
- Avogadro's Number: Explanation of constant , representing the number of entities in one mole.
500K+ Students Use These Powerful Tools to Master Law of Conservation of Mass For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
234 flashcards
Flashcards on Law of Conservation of Mass
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards27 quizzes
Quizzes on Law of Conservation of Mass
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes21 questions
Exam questions on Law of Conservation of Mass
Boost your confidence with real exam questions.
Try Chemistry Questions1 exams created
Exam Builder on Law of Conservation of Mass
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Law of Conservation of Mass
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Law of Conservation of Mass you should explore
Discover More Revision Notes Related to Law of Conservation of Mass to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Chemical Reactions and Stoichiometry
Chemical Reactions Quantitative Calculations
214+ studying
197KViews