Photo AI
Last Updated Sep 24, 2025
Concentration of Solutions Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Concentration of Solutions quickly and effectively.
266+ students studying
Concentration of Solutions
Definition and Measurement
Determining solution concentrations is essential for obtaining precise experimental results. Techniques such as Titration and Spectroscopy are commonly employed in laboratories to measure and analyse these concentrations. Understanding concentration measurements is equally important in daily contexts, from laboratory settings to culinary adjustments.
Definition of Concentration
- Concentration: Indicates the quantity of solute present within a given volume of solvent or solution.
- Units of Concentration: Commonly expressed as molarity, measured in moles per litre (M).
- Concentration: The quantity of solute per unit volume.
Explanation of Molarity
- Molarity Formula: Defined as:
- Where represents the moles of solute
- denotes the solution volume in litres
Worked Example: Calculating Molarity
- Problem: Determine the molarity of a solution with 2 moles of solute in 1 litre of solution.
- Identify moles of solute:
- Identify volume of solution:
- Compute molarity:
Comparison of Concentrated vs. Dilute Solutions
- Concentrated Solutions: High density of solute particles.
- Dilute Solutions: Lower density of solute particles.

Different Ways to Measure Concentrations
Molarity
- Definition: Amount of solute in moles per litre of solution.
- Example: Creating a 1 mol/L NaCl solution.
Mass Percentage
- Definition: Mass of solute compared to the mass of the solution, expressed as a percentage.
- Example: Making sure a beverage contains 12% sugar.
Parts Per Million (PPM)
- Definition: Measurements for very dilute concentrations, such as pollutants.
- Example: Monitoring water quality with chlorine levels at 4 ppm.
Normality
- Explanation: Utilised in titration, corresponds to the amount of equivalent substances.
- Example: Titrating sulphuric acid with sodium hydroxide.
Importance of Understanding Concentration
-
Applications in Science:
- Vital for accuracy in:
- Laboratory experiments
- Pharmaceutical formulations
- Industrial processes
- Vital for accuracy in:
-
Real-world Impact:
- Essential for medication effectiveness and maintaining food safety standards.
Comprehending concentration is crucial for safeguarding public health and safety.
Introduction to Dilutions
-
Dilution: The process of decreasing the concentration of a solute by adding more solvent.
-
Key Principles: The quantity of solute remains constant during dilution, adhering to the conservation of mass principle.
-
Formula:
Example of Single-step Dilution
- Problem: Dilute 50 mL of 1 M HCl to 200 mL.
- Solution:
Example of Multi-step Dilution
- Problem: Dilute in two steps: First to 250 mL, then to 500 mL.
- Solution:
- Step 1: Dilute to 250 mL.
- Step 2: Further dilute to 500 mL.
- Step 1: Dilute to 250 mL.
Introduction to Calculation Formula
- Concentration : Solute amount per solution volume (mol/L).
- Number of Moles : Quantity of solute measured in moles.
- Volume : Total space occupied by the solution in litres.
Example Calculations
-
Determining Concentration:
- Given: 0.5 moles in 2 litres
- Solution:
-
Determining Moles:
- Formula:
- Example: If M, L
- Solution:
- Formula:
-
Determining Volume:
- Formula:
- Example: If mole, mol/L
- Solution:
- Formula:

Titration Method
Definition
- Titration: A method for determining concentration using a titrant of known concentration.
Step-by-Step Guide
- Preparation: Prepare solutions of titrant and analyte.
- Procedure: Add titrant to analyte, observing the colour change at the endpoint.
- Example Calculation:
- 25 mL of 0.1 M titrant neutralises 50 mL of an analyte.
- Use formula:
- Solution:
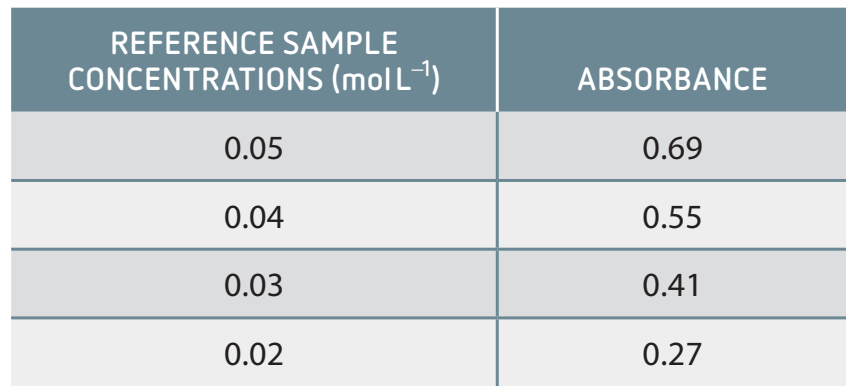
Application of Concentration Measurements in Lab Settings
-
Standard Solutions: Designed with precise measurements using volumetric flasks, analytical balances, and pipettes.
-
Spectroscopy: Determines concentration through light absorbance.
- Procedure: Prepare and measure using calibration standards.
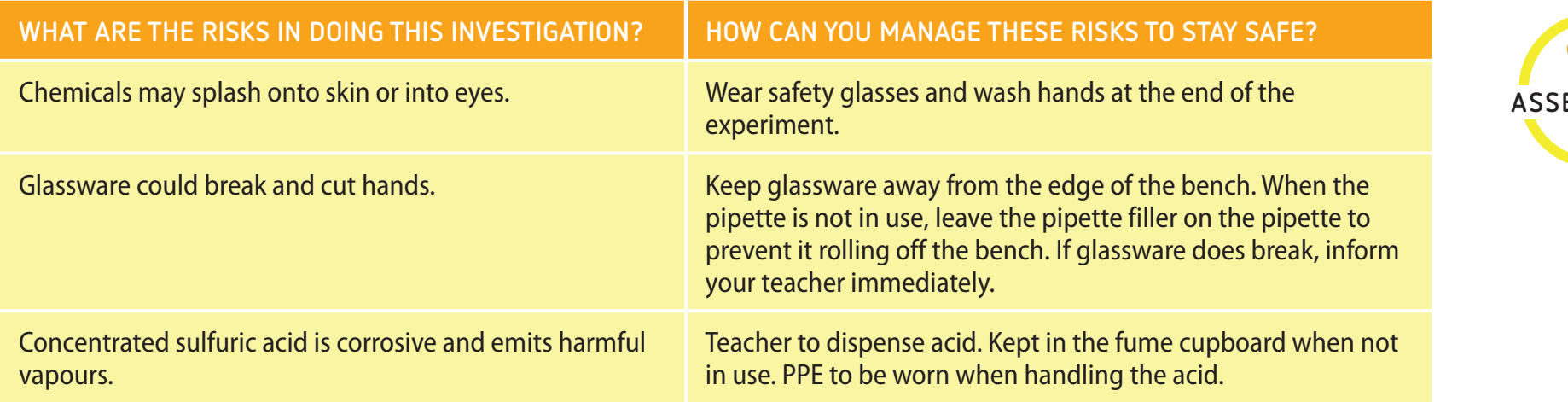
Safety Protocols for Laboratory Work
- PPE: Lab coats, gloves, and goggles.
- Risk Assessment: Effective management of hazards.
- Case studies demonstrate how the correct use of PPE has saved lives.
Best Practices
- Calibration: Ensure volumetric glassware and analytical balances are verified before usage.
Conclusion
Grasping and accurately measuring concentration is foundational for a myriad of applications in both the scientific community and daily life. Proficiency in these methods ensures exactness and reliability in analytical chemistry, enriching both educational pursuits and practical problem-solving experiences.
500K+ Students Use These Powerful Tools to Master Concentration of Solutions For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
225 flashcards
Flashcards on Concentration of Solutions
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards22 quizzes
Quizzes on Concentration of Solutions
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes24 questions
Exam questions on Concentration of Solutions
Boost your confidence with real exam questions.
Try Chemistry Questions2 exams created
Exam Builder on Concentration of Solutions
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Concentration of Solutions
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Concentration of Solutions you should explore
Discover More Revision Notes Related to Concentration of Solutions to Deepen Your Understanding and Improve Your Mastery
