Photo AI
Last Updated Sep 24, 2025
Solutions and Concentration Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Solutions and Concentration quickly and effectively.
457+ students studying
Solutions and Concentration
Introduction to Solutions
- Solution: A homogeneous mixture with evenly mixed substances at the molecular or ionic level.
- Significance: Solutions are essential for chemical reactions and various biological and industrial processes.
Distinguishing Solutions, Suspensions, and Colloids
- Solution: Homogeneous with molecules dissolved at a molecular level (e.g., sugar in water).
- Suspension: Heterogeneous with larger particles that settle over time (e.g., muddy water).
- Colloid: Contains intermediate particle sizes that do not settle (e.g., whipped cream).
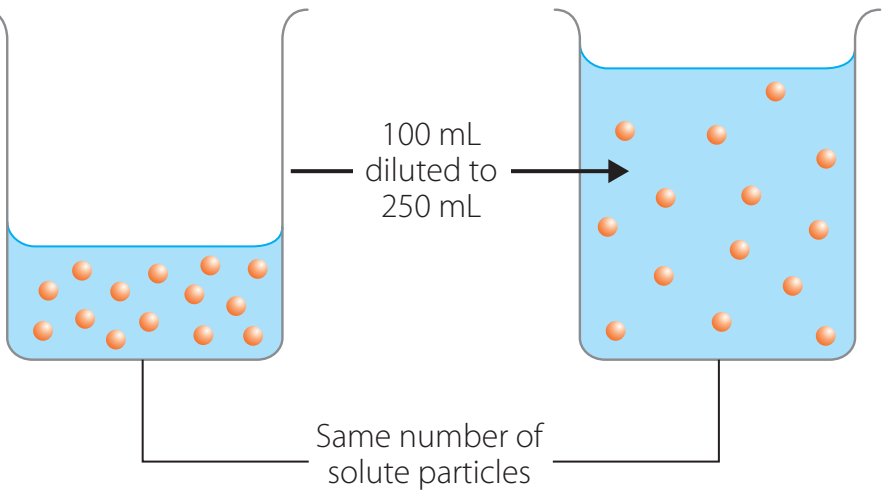
The diagram shows differences in particle distribution.
-
Settling:
- Solutions: Particles remain evenly distributed.
- Suspensions: Particles settle over time.
- Colloids: Particles remain dispersed without settling.
-
Light Interactions:
- Solutions: Appear clear with no light scattering.
- Suspensions: Appears cloudy as particles block light.
- Colloids: Exhibit the Tyndall effect, scattering light, giving a foggy look.

To observe the Tyndall effect, shine a torch through an aerosol mist.
Methods of Separation
- Filtration:
- Suspensions: Can be separated by filtration.
- Solutions: Cannot be filtered; components are uniformly mixed.
FAQ: Solutions cannot be filtered because of molecular mixing.
Components of a Solution
-
Solution: A homogeneous mix of solute and solvent.
-
Components:
- Solute: Present in a lesser amount and is the substance being dissolved (e.g., sugar in tea).
- Solvent: Present in a greater amount and dissolves the solute (e.g., water).
-
Roles and States:
- Solute: Affects characteristics such as taste and pH.
- Solvent: Facilitates chemical reactions and influences the physical state.
- State Examples:
- Solid in Liquid (e.g., salt in water)
- Gas in Liquid (e.g., CO₂ in soda)
- Liquid in Liquid (e.g., ethanol in water)
Solubility and Molecular Interactions
-
Intermolecular Forces:
- Hydrogen Bonds: Vital for solvents like water.
- Van der Waals: Play a role in non-polar compounds.
- Dipole-Dipole: Affect solubility among polar molecules.
-
Solute Distribution:
- Even solute distribution leads to a homogeneous mixture.

Solubility Effects
-
Temperature:
- Solids: Solubility generally increases with temperature.
- Gases: Solubility typically decreases as temperature rises.
-
Pressure:
- Impacts gas solubility in liquids; increasing pressure typically increases solubility.
-
Molecular Polarity:
- "Like dissolves like": Polar solvents dissolve polar solutes effectively.

Solubility is crucial in practical applications like drug formulation and culinary practices.
Observing and Measuring Solution Concentration
-
Molarity:
- Definition: Moles of solute per litre of solution.
- Formula:
-
Methods:
- Percentage Concentration (% m/v): Mass of solute per 100 mL of solution.
- Parts per Million (ppm): Mass of solute per million parts of solution.
Conducting Titrations
- Process:
- Fill a burette with titrant.
- Add titrant to analyte until the endpoint is reached.
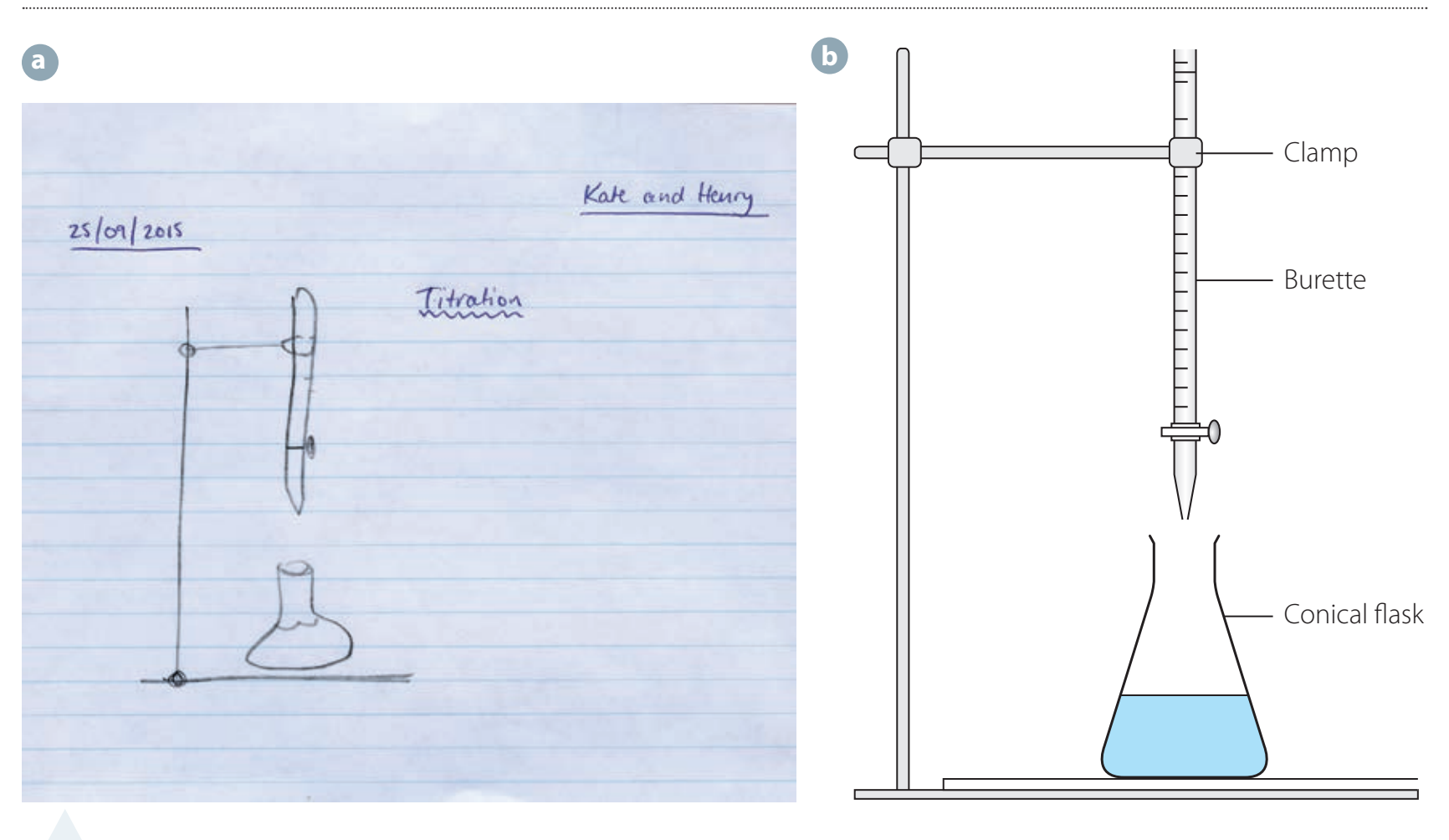
Common Errors:
- Misreading the meniscus
- Using an incorrect indicator
- Ensure accurate measurements and avoid contamination.
Sample Problem: Calculating Molarity
Example: Calculate the molarity of a solution containing 10 moles of solute in 5 litres of solution.
Solution:
- Use the molarity formula:
- Substitute the values:
- Therefore, the molarity of the solution is 2 mol/L (or 2M).
Example: Calculate the percentage concentration (% m/v) of a solution containing 5g of salt in 250mL of solution.
Solution:
- Use the formula: % m/v =
- Substitute the values: % m/v =
- Therefore, the percentage concentration is 2% m/v.
Key Takeaway
- Consistent practice enhances understanding of solution concentrations.
Summary and FAQ
- Main Concepts:
- Factors affecting solubility include temperature, pressure, and molecular interactions.
To effectively solve practical problems, an understanding of concentration and solubility principles is vital.
500K+ Students Use These Powerful Tools to Master Solutions and Concentration For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
225 flashcards
Flashcards on Solutions and Concentration
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards22 quizzes
Quizzes on Solutions and Concentration
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes24 questions
Exam questions on Solutions and Concentration
Boost your confidence with real exam questions.
Try Chemistry Questions2 exams created
Exam Builder on Solutions and Concentration
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Solutions and Concentration
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Solutions and Concentration you should explore
Discover More Revision Notes Related to Solutions and Concentration to Deepen Your Understanding and Improve Your Mastery
