Photo AI
Last Updated Sep 24, 2025
Bond Energy and Enthalpy Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Bond Energy and Enthalpy quickly and effectively.
326+ students studying
Bond Energy and Enthalpy
Introduction
Calculating enthalpy change (ΔH) through bond energies helps determine whether a reaction absorbs or releases energy. This understanding is essential for evaluating fuel efficiency and forecasting reaction outcomes.
Enthalpy (ΔH): Represents the total heat content of a system, indicating the energy absorbed or released during a reaction.
Key Concepts:
- Average Bond Energies: Vital for precise calculations.
- Sign Conventions:
- Energy is required for breaking bonds (positive).
- Energy is released during bond formation (negative).
Definitions and Distinctions
- Bond Energy: Energy needed to break 1 mole of bonds in the gaseous state, expressed in kJ/mol.
- Bond Enthalpy: Specific energy needed to break a certain bond within a molecule under standard conditions.
- Bond Dissociation Energy: Positive energy value required to separate a bond into individual atoms.
- Bond Energy: Uniform energy to dissociate 1 mole of a chemical bond.
- Bond Enthalpy: Relates to breaking a specific bond in its standard state.
- Bond Dissociation Energy: Always positive as energy is necessary to break a bond, highlighting its importance.
Conceptual Clarifications
-
Illustrative Diagrams:
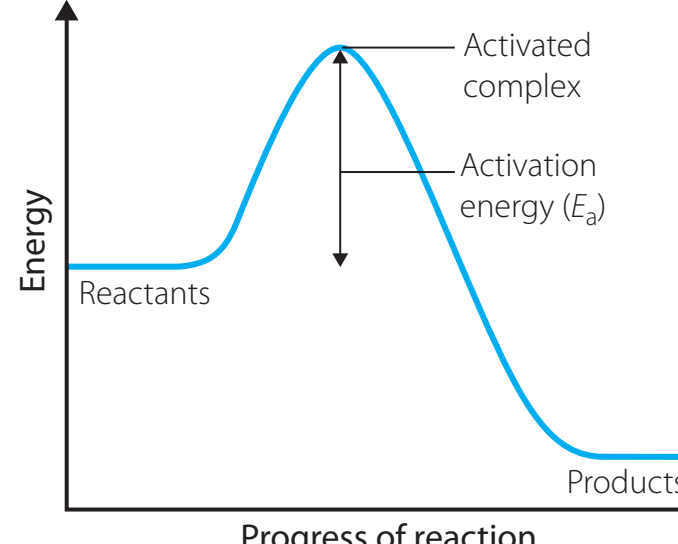
- Visualising the energy absorption during hydrogen molecule (H₂) dissociation aids in comprehending energy state transitions effectively.
- Question: "At what stage does energy absorption occur in this process? Clearly annotate these phases."
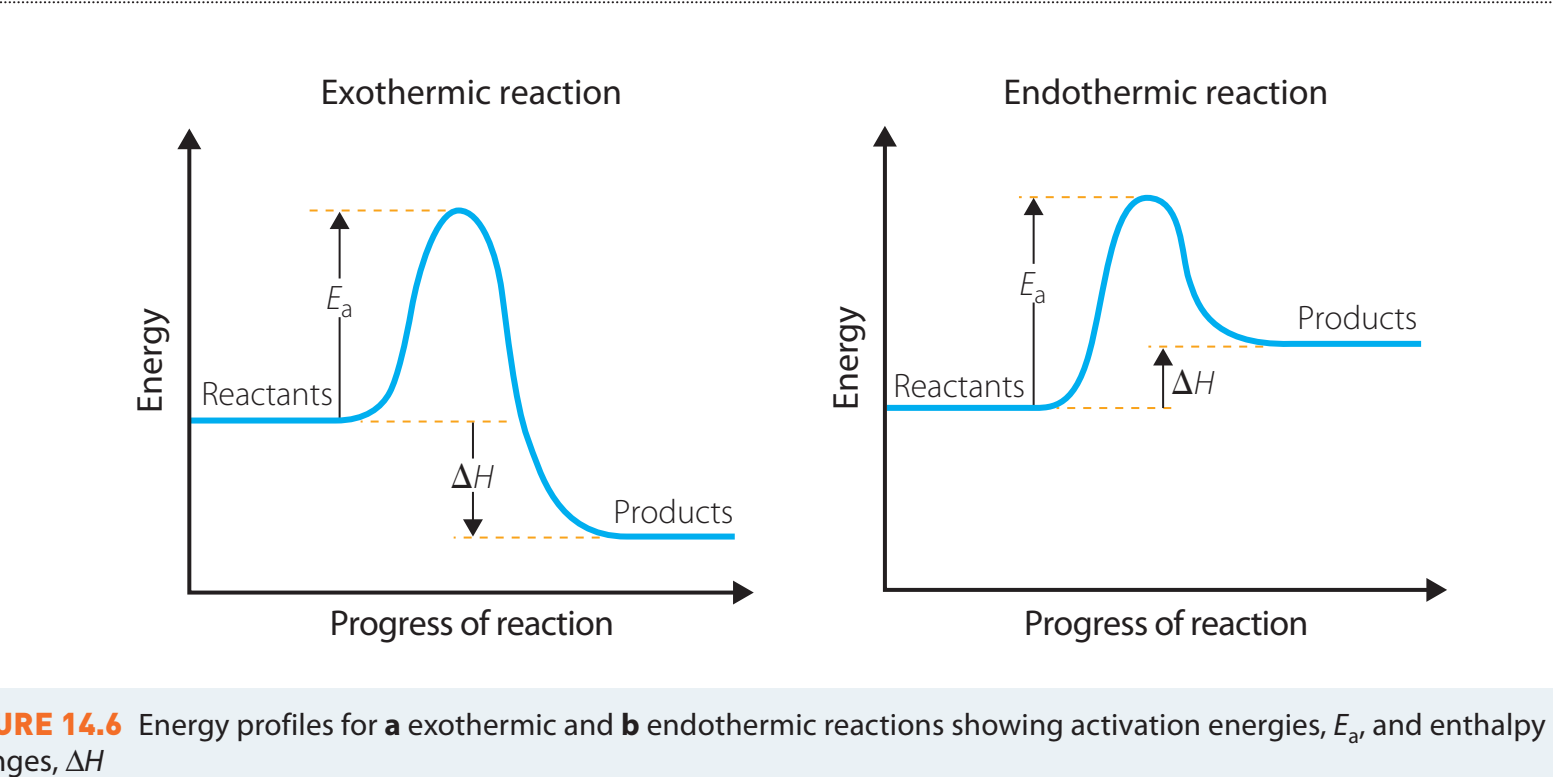
- This diagram contrasts the energy necessary for breaking bonds, providing insight into energy dynamics for learners.
Enthalpy Change Calculation
-
Calculation Steps:
- Calculate the energy required to break bonds in reactants.
- Subtract the energy released during product formation.
-
Formula:
-
Sign Conventions:
- Positive ΔH: Indicative of endothermic reactions.
- Negative ΔH: Indicative of exothermic reactions.
Enthalpy Change (ΔH): The comprehensive energy exchange within a reaction, determined using bond energies.
Worked Example: Hydrogen Dissociation
- Step-by-Step Calculation:
- Step 1: Evaluate the initial bond energy of H₂ = 436 kJ/mol.
- Step 2: Compute the total energy input required for the bond dissociation:
- Given energy change for H₂ → 2H = 436 kJ/mol.
Each C-H bond is approximately 413 kJ/mol, O-H is about 463 kJ/mol, and H-O-H is around 498 kJ/mol. Explain the net energy absorbed or released by detailing each step in the process of breaking and forming bonds.
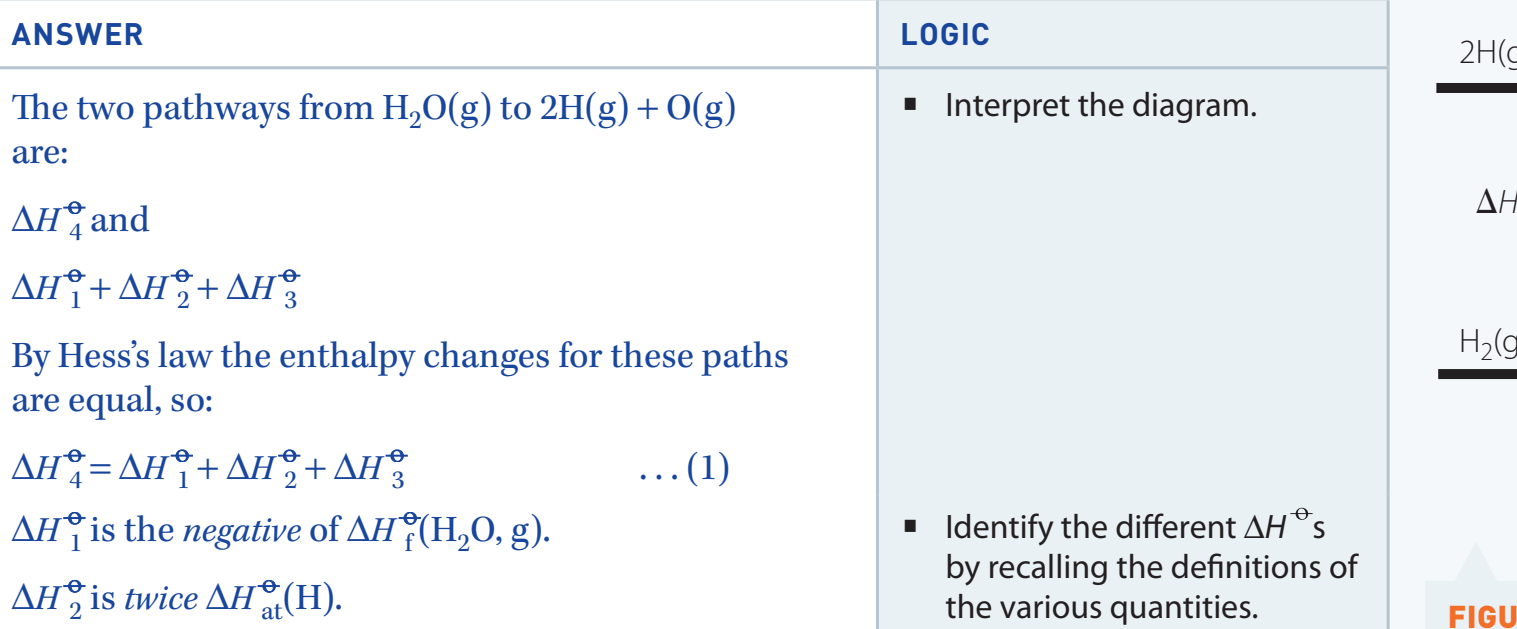
- Focus: Observe the energy transition from reactants to products, highlighting pathways of exothermic and endothermic reactions.
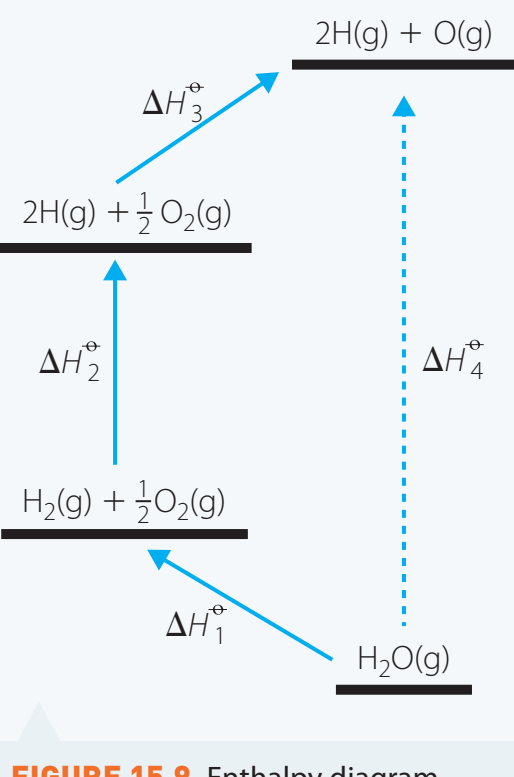
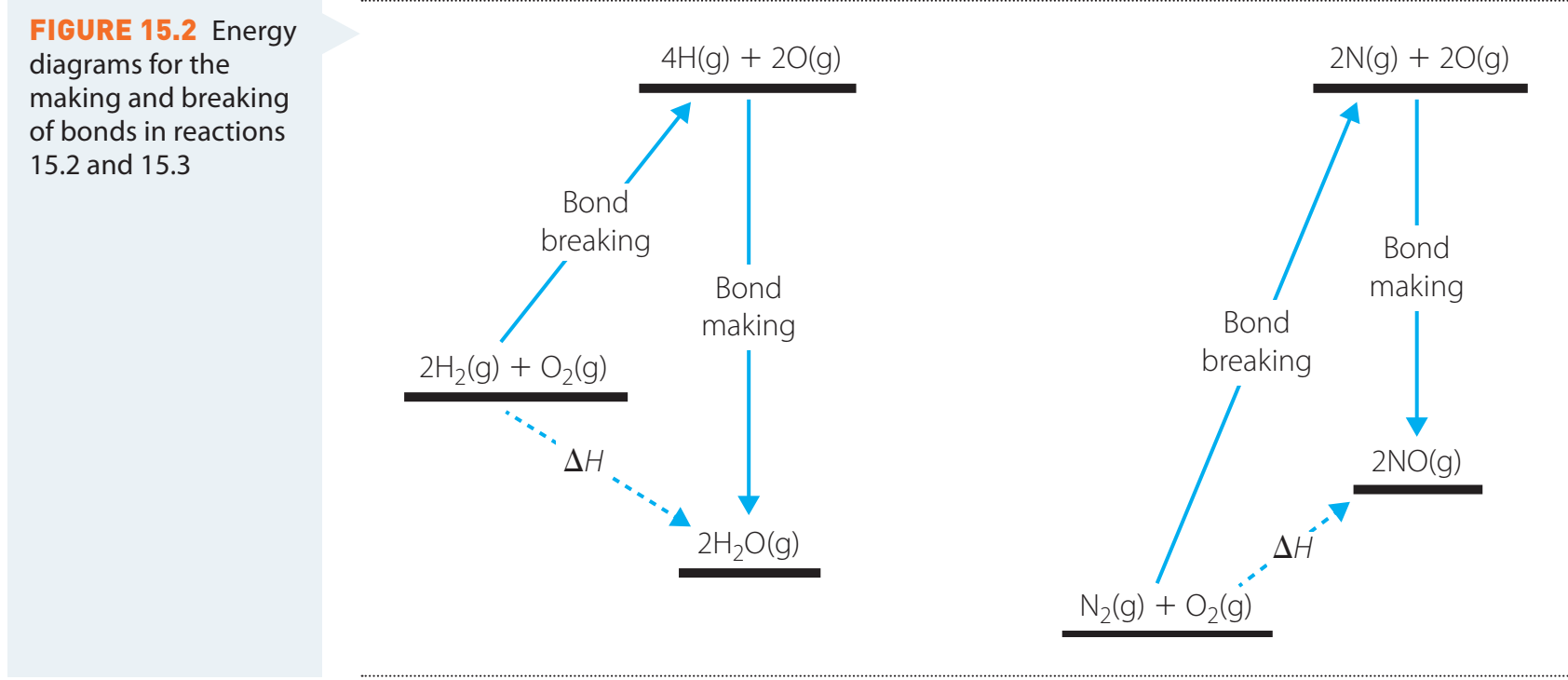
Hess's Law Cycle
- Focus: Illustrates the conservation of energy and consistent enthalpy independent of the pathway.
Hess's Law Application: Different pathways result in identical enthalpy changes, emphasising energy conservation.
- Example: Compare pathways to validate equal enthalpy changes.
Factors Influencing Bond Energies
-
Factors:
- Molecular Context: Influences from neighbouring atoms or groups affect bond strength and energy.
- Temperature: Changes in temperature can affect bond energies and consequently impact reaction rates.
- Pressure: Variations in pressure can influence the energy requirements for altering bonds.
-
Practical Implications:
- Combustion: Energy considerations are vital for determining fuel efficiency in engines.
- Biological Systems: Enzyme-mediated reactions demonstrate variations in energy demands.
Applications and Examples
- Average Bond Energies:
- Recognise variations due to molecular environments, essential for comparing chemical reactions.
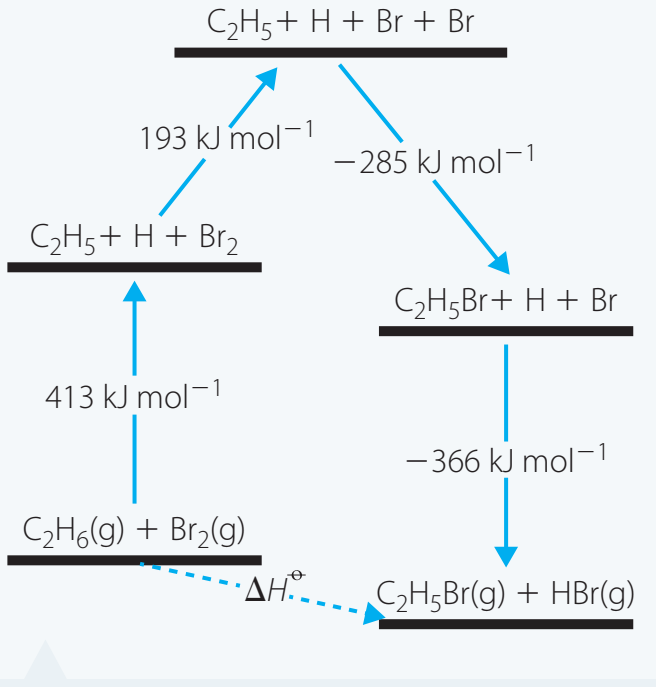
- Worked Example: Ethane + Bromine Reaction
-
Step 1: Identify reactant bonds:
- C—C, C—H in ethane.
- Br—Br in bromine.
-
Step 2: Compute the total energy needed for breaking these bonds:
- C—C: 346 kJ/mol
- C—H: 6 × 413 kJ/mol = 2478 kJ/mol
- Br—Br: 193 kJ/mol
-
Total Energy for Breaking: 346 + 2478 + 193 = 3017 kJ/mol
-
Step 3: Consider the formation of new bonds:
- New bonds such as C—Br influence the final ΔH.
-
Practice Exercises
-
Exercise 1: Calculate ΔH for propane (C₃H₈) combustion.
- Solution:
- Bonds broken in C₃H₈: 2(C-C) + 8(C-H) = 2(346) + 8(413) = 692 + 3304 = 3996 kJ/mol
- Bonds broken in O₂: 5(O=O) = 5(498) = 2490 kJ/mol
- Total energy for breaking bonds = 3996 + 2490 = 6486 kJ/mol
- Bonds formed in CO₂: 6(C=O) = 6(799) = 4794 kJ/mol
- Bonds formed in H₂O: 8(O-H) = 8(463) = 3704 kJ/mol
- Total energy for forming bonds = 4794 + 3704 = 8498 kJ/mol
- ΔH = 6486 - 8498 = -2012 kJ/mol (exothermic)
- Solution:
-
Exercise 2: Ascertain ΔH for ammonia (NH₃) synthesis from nitrogen (N₂) and hydrogen (H₂).
- Solution:
- Bonds broken: N≡N + 3(H-H) = 946 + 3(436) = 946 + 1308 = 2254 kJ/mol
- Bonds formed: 3(N-H) = 3(391) = 1173 kJ/mol
- ΔH = 2254 - 1173 = 1081 kJ/mol (endothermic)
- Solution:
Avoid common mistakes such as incorrect formula usage or omitting bond counts. Ensure accurate accounting of each bond involved.
Additional Examples
- Methane (CH₄) Combustion offers insight into common combustion reaction energetics.
- Hydrazine (N₂H₄) Reactions illustrate more complex nitrogen configurations.
Limitations of Using Average Bond Energies
Understanding the limitations of using average bond energies is essential for precise chemical predictions. Theoretical calculations often diverge from experimental results.
Variability Due to Environmental Factors
- Phase state: Gas, liquid, and solid states influence bond energies distinctly.
- Temperature: Fluctuations can alter bond strength.
- Pressure: Changes can impact physical conditions affecting bonds.
Environmental conditions can create considerable variability in bond energies, necessitating consideration of these factors during calculations.
Non-Uniformity of Bond Energies
- Bond energies are not consistent across different compounds.
- For instance, C—H bond energy differs substantially in methane compared to ethane.
Bond Energy Non-Uniformity: Identical types of bonds in different molecules can exhibit varying bond energies.
Strategic Tips for Simplification and Avoiding Errors
- Sign Conventions: Remember, bond breaking is endothermic (+), whereas bond forming is exothermic (−).
- Identify errors such as incorrect bond energy values or miscalculations of the total bonds involved.
Visual and Interactive Aids
- Flowcharts & Guides:
Always present step-by-step calculations with justification for each step to ensure transparency and understanding.
This comprehensive revision note ensures a solid understanding of enthalpy and bond energy concepts essential for chemistry students gearing up for exams. By grasping calculations, limitations, and practical applications, students will enhance their chemical knowledge and readiness for assessments.
500K+ Students Use These Powerful Tools to Master Bond Energy and Enthalpy For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
241 flashcards
Flashcards on Bond Energy and Enthalpy
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards24 quizzes
Quizzes on Bond Energy and Enthalpy
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes16 questions
Exam questions on Bond Energy and Enthalpy
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Bond Energy and Enthalpy
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Bond Energy and Enthalpy
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Bond Energy and Enthalpy you should explore
Discover More Revision Notes Related to Bond Energy and Enthalpy to Deepen Your Understanding and Improve Your Mastery
