Photo AI
Last Updated Sep 24, 2025
Chemistry - Enthalpy Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Chemistry - Enthalpy quickly and effectively.
492+ students studying
Chemistry - Enthalpy
Overview
A thorough understanding of heat of combustion and enthalpy of formation is fundamental in chemistry. These concepts are pivotal for enhancing energy efficiency and supporting sustainable practices, contributing to more effective energy production and promoting environmentally friendly processes.
Definition of Key Terms
- Heat of Combustion: The thermal energy released when one mole of a substance combusts completely in the presence of excess oxygen under standard conditions.
- Standard Enthalpy of Formation (ΔH): The change in energy during the formation of one mole of a compound from its constituent elements in their standard states.
- Heat of Combustion: Essential for calculating energy yields, optimising fuel efficiency, and assessing environmental impacts.
- Enthalpy of Formation (ΔH): Integral for evaluating reaction enthalpies in chemical thermodynamics.
Measurement Techniques
- Calorimetry: The primary technique for measuring heat of combustion.
- Bomb Calorimeter:
- Key Components: Ignition coil, oxygen bomb, water jacket, and thermometer.
- Steps:
- Insert the sample into the oxygen bomb.
- Secure the bomb within the water jacket.
- Initiate combustion using the ignition coil.
- Record the temperature change with the thermometer.
- Bomb Calorimeter:
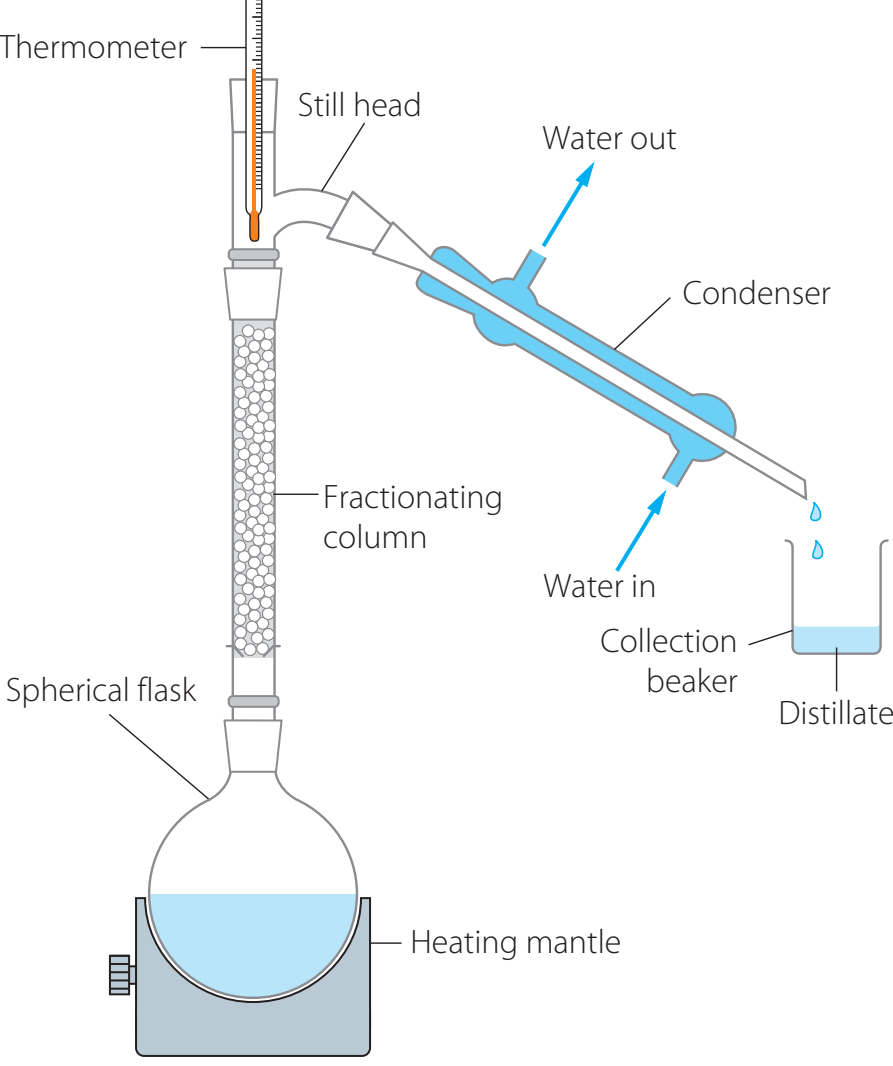
Temperature Measurement Details
- Formula:
- m: mass
- c: specific heat capacity
- ΔT: temperature change
Hess's Law
Hess's Law states that the total enthalpy change of a chemical reaction is independent of the pathway taken, consistent with the law of conservation of energy.
- Formula:
It is crucial to maintain consistency in units to avoid errors while applying Hess's Law.
Worked Examples
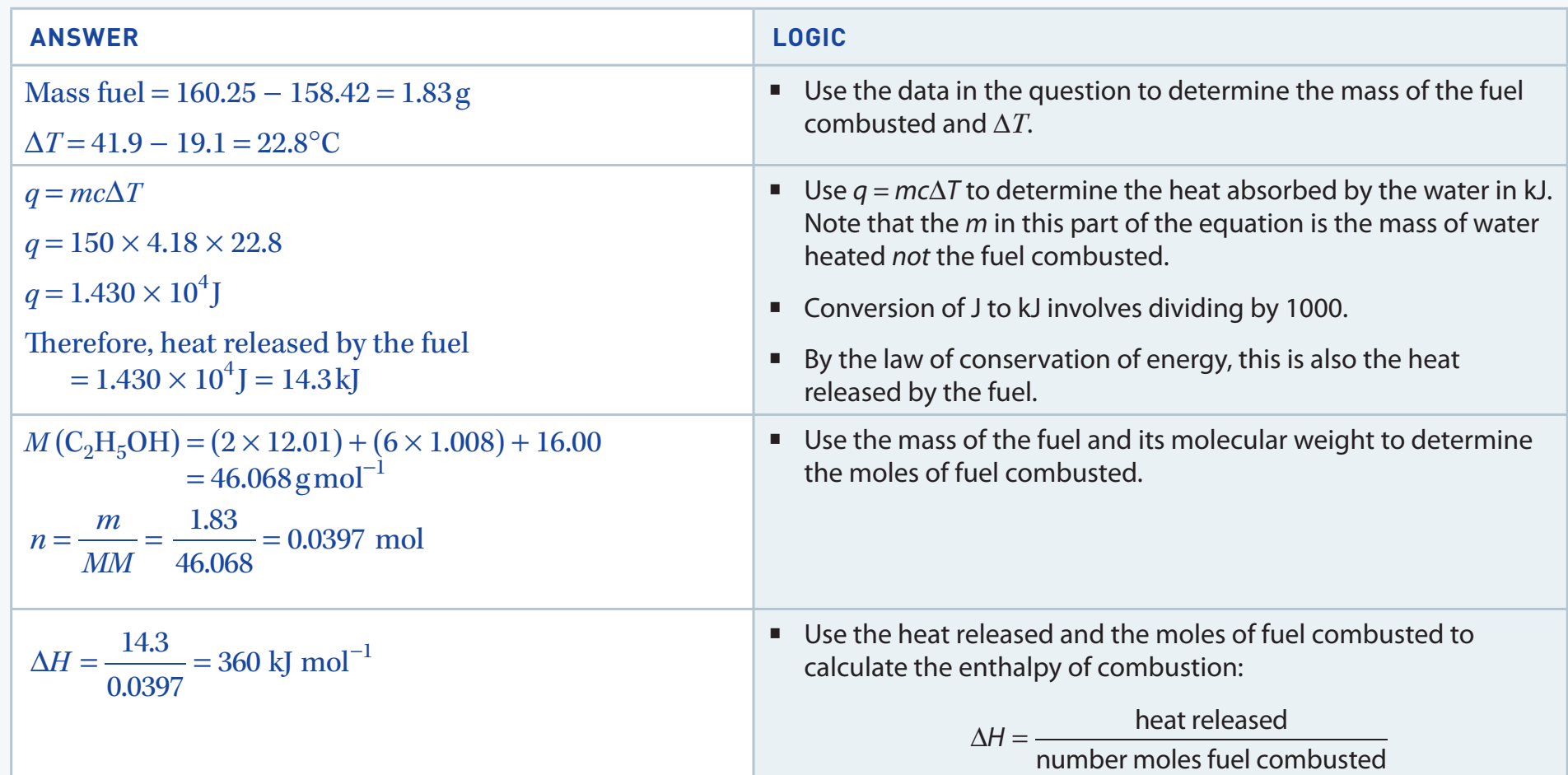
Example 1: Methane Combustion
The combustion of methane is represented by the equation:
- Calculation of :
- Apply the formula:
- Apply the formula:
Example 2: Calculating Enthalpy of Formation of CO₂
Given Data:
- Heat of combustion of carbon:
- Heat of combustion of hydrogen:
Objective: Determine the enthalpy of formation for carbon dioxide using Hess's Law.
Solution:
-
Write the combustion equation for carbon: This directly gives us the enthalpy of formation of CO₂:
-
The enthalpy of formation of CO₂ is equal to its heat of combustion because carbon and oxygen are both in their standard states.
Assumptions and Limitations
Recognising the limitations and assumptions is essential when using heats of combustion to evaluate enthalpies of formation.
- Complete Combustion: Assumes all fuel is entirely converted to CO₂ and H₂O, which might not always be achievable in practice.
- Constant Conditions: Assumes standard conditions of 1 atm and 25°C, which may not always be relevant in different contexts.
- Perfect Insulation: Assumes no heat is lost, though slight heat losses can occur.

Practice Problem
Problem: Determine the enthalpy of formation of compound Z from substances X and Y using combustion data.
- Steps:
- Decompose into individual reactions.
- Apply Hess's Law to perform the calculations.
Solution: Given the combustion data for substances X, Y, and Z:
- Heat of combustion of X: -200 kJ/mol
- Heat of combustion of Y: -300 kJ/mol
- Heat of combustion of Z: -400 kJ/mol
Using Hess's Law:
- Write all combustion reactions
- Manipulate equations to isolate formation of Z
- Calculate: ΔH(formation Z) = ΔH(combustion X) + ΔH(combustion Y) - ΔH(combustion Z)
- ΔH(formation Z) = -200 + (-300) - (-400) = -100 kJ/mol
Correction Methods
- Improved Insulation: Utilise superior materials to minimise heat loss.
- Precise Calorimeter Calibration: Ensure precise settings and regular calibration checks for accuracy.
Exam Tips
- Sign Conventions are essential when reversing reactions.
- Verify that each intermediate step correctly leads to the final equation.
Visual Learning
Engaging with diagrams can significantly enhance comprehension.
500K+ Students Use These Powerful Tools to Master Chemistry - Enthalpy For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
241 flashcards
Flashcards on Chemistry - Enthalpy
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards24 quizzes
Quizzes on Chemistry - Enthalpy
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes16 questions
Exam questions on Chemistry - Enthalpy
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Chemistry - Enthalpy
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Chemistry - Enthalpy
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Chemistry - Enthalpy you should explore
Discover More Revision Notes Related to Chemistry - Enthalpy to Deepen Your Understanding and Improve Your Mastery
