Photo AI
Last Updated Sep 24, 2025
Enthalpy and Hess's Law Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Enthalpy and Hess's Law quickly and effectively.
219+ students studying
Enthalpy and Hess's Law
Definition of Hess's Law
Imagine navigating a city using different routes, each leading to the same destination. This concept illustrates Hess's Law in chemistry.
Definition of Hess's Law
Hess's Law: Hess's Law asserts that the total enthalpy change for a chemical reaction remains constant, regardless of the pathway taken, provided the initial and final states are identical.
Hess's Law is beneficial in predicting energy transformations in chemical reactions through various pathways.
Mathematical Expression
Hess's Law can be mathematically expressed as:
- : Total enthalpy change.
- : Enthalpy changes for individual steps.
This equation indicates that the sum of all individual step enthalpies results in the total enthalpy change.
-
Utility of Summation: In multi-step syntheses, verifying the total enthalpy change is essential for assessing reaction efficiency.
-
Example Calculation:
- Result: kJ/mol.
Visualisation through Diagrams
Below are diagrams illustrating how different reaction pathways can produce the same total enthalpy changes:
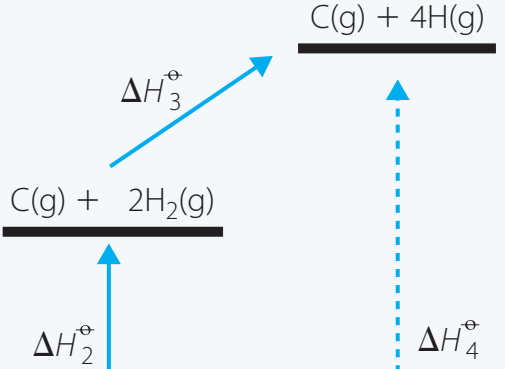
- Diagram Explanation: Depicts multiple routes from graphite to diamond to visually affirm that energy conservation holds.
Principle of Energy Conservation
- Hess's Law is grounded in the law of conservation of energy.
- Energy cannot be created or destroyed, it can only be transformed.
Understanding that energy only transforms and is neither created nor destroyed is fundamental to grasping Hess's Law as an aspect of energy conservation.
Introductory Example
Explore the enthalpy change in water decomposition:
- Initial Condition: Water decomposes into hydrogen and oxygen.
- Pathways Options: Calculate enthalpies for different routes.
- Numerical Details:
- Route A: kJ/mol.
- Route B: kJ/mol.
- Final Computation:
- Outcome: kJ/mol parallels direct decomposition.
Interactive Engagement
Reinforce understanding with a practice task:
- Quick Quiz: Calculate the enthalpy change for a specified multi-step reaction.
- Problem: Determine the total given steps of kJ/mol.
- Solution: kJ/mol
- Use the equation to compute the sum.
Engaging with these concepts empowers students with a thorough understanding of Hess's Law, pivotal for both academic and practical chemistry applications.
Introduction to Standard Enthalpy Changes
-
Standard Enthalpy of Formation: Enthalpy change when one mole of a compound forms from its elements in their standard states.
-
Standard Enthalpy of Combustion: Enthalpy change when one mole of a substance fully combusts in oxygen.
-
Standard Enthalpy of Reaction: Enthalpy change for a reaction under standard conditions.
-
Significance: Critical for understanding energy variations in reactions, key to thermodynamic computations.
1. Key Formula and Calculations
-
Formula: Reaction enthalpy calculation:
-
Calculation Process:
- Determine the standard enthalpy of formation for each reactant and product.
- Compute the total enthalpy for products and reactants separately.
- Subtract the total enthalpy of reactants from that of products to find the reaction enthalpy.
- Key actions: Correctly identify and subtract enthalpy values.
2. Referencing Standard Enthalpy Data
- Utilising Tables:
- Tables provide standard enthalpy values for various substances; understanding table layout is essential.
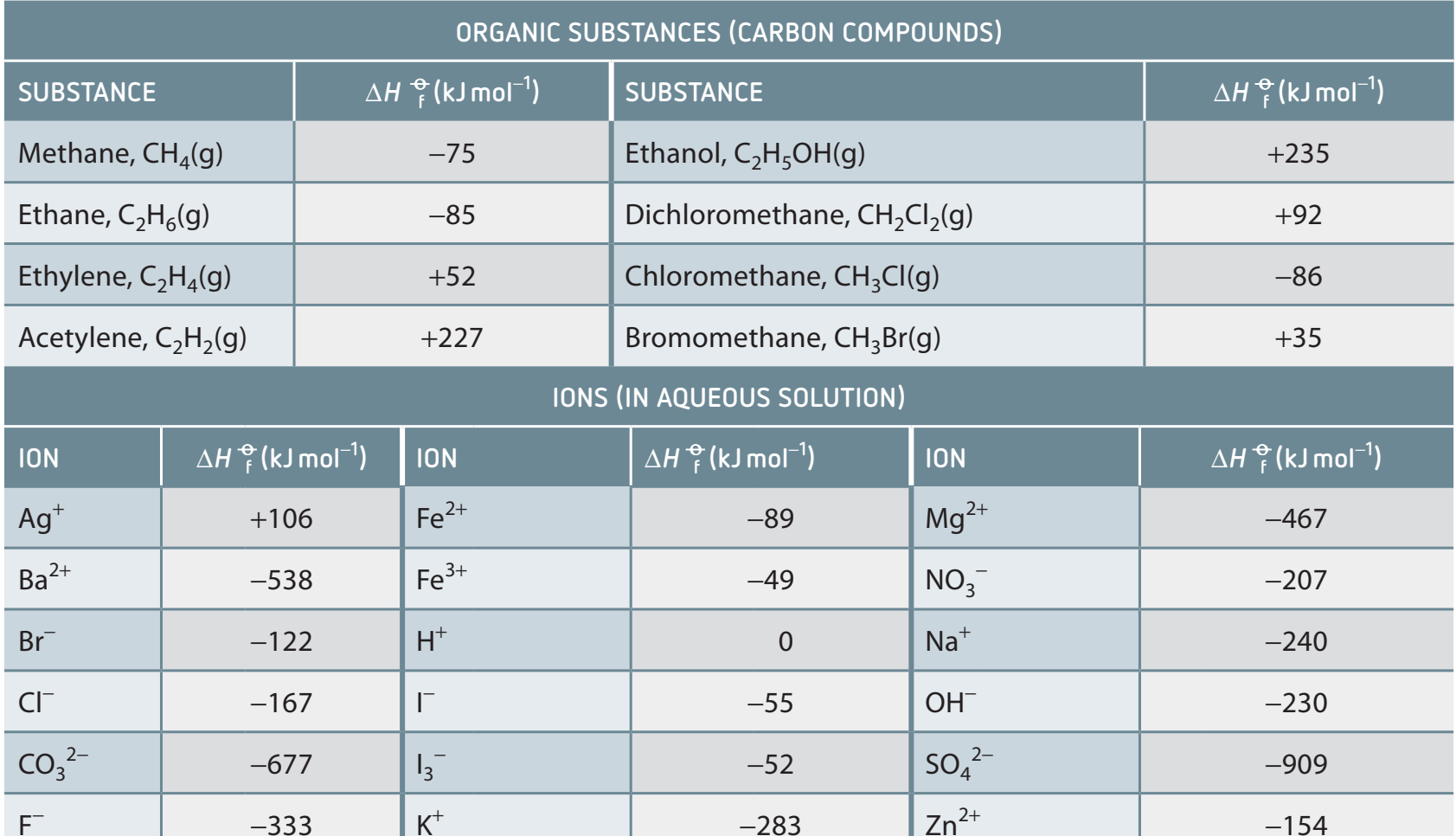
- Familiarisation:
- Rapid data retrieval is crucial for efficient problem-solving.
3. Visualisation for Comprehension
-
Energy Level Diagram:
- Shows how reactants transform into products, indicating energy changes.
-
Role of diagrams: Diagrams help visualise enthalpy changes, offering insights beyond numerical data.
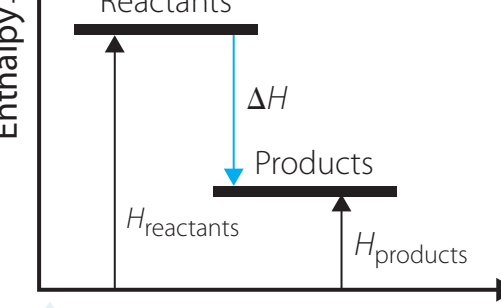
4. Practical Example
-
In-depth Calculation Process:
- Step-by-step Breakdown for the combustion of Methane:
- Find the enthalpy of formation for and .
- Identify the enthalpy of formation for .
- Substitute these values into the formula for reaction enthalpy.
- Calculate total enthalpies and perform subtraction to obtain results.
- Step-by-step Breakdown for the combustion of Methane:
-
Numerical Example:
- Standard enthalpies:
- Calculation:
- Products:
- Reactants:
- Standard enthalpies:
Bond Energy Data and Calculations
What are Bond Energies?
Bond Energies: The energy required to break one mole of bonds in gaseous molecules, indicating bond strength.
Formula for Enthalpy Change
To determine enthalpy change () using bond energies, the formula is:
Key Concepts: Average vs Real Bond Energies
-
Average Bond Energies:
- Reflect general bond strengths.
- May vary with specific chemical environments.
-
Real Bond Energies:
- Specific to certain molecules.
- Offer greater accuracy than averages.
Recognise that averages are broad estimates. Specific circumstances can significantly adjust bond strengths.
Presentation of Data
Common Bond Energies
| Bond Type | Average Bond Energy (kJ/mol) |
|---|---|
| C-H | 413 |
| C-C | 348 |
| O=O | 498 |
Example: Calculating Reaction Enthalpy
For the decomposition of hydrogen peroxide, :
-
Reactant Bonds:
- 4 O-H and
- 2 O-O bonds
- Total energy:
- = 1652 + 996 = 2648 kJ/mol
-
Product Bonds:
- 4 O-H and
- 1 O=O bond
- Total energy:
- = 1652 + 498 = 2150 kJ/mol
-
Enthalpy Change:
- kJ/mol
**Diagrams
- Bond Breaking and Forming Process**
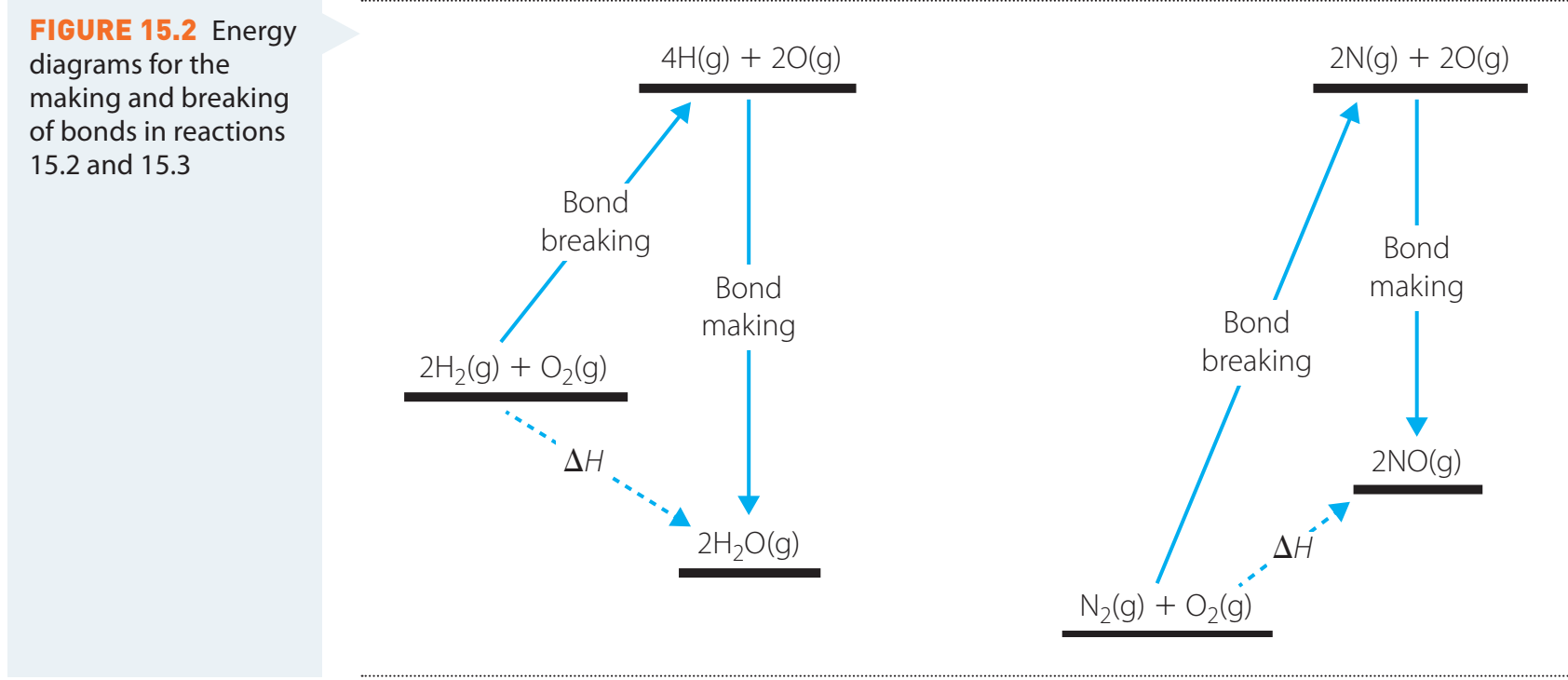
Advantages and Limitations
Advantages of Bond Energy Data:
- Simple to use for estimates.
- Directly related to bond-breaking processes.
Limitations:
- Less accurate than standard enthalpies.
- Average values may not accurately represent specific bonds.
Practical Considerations
When to Use Bond Energies:
- Estimating energy trends when detailed data is unavailable.
- Educational purposes to demonstrate bond strength and energy relationships.
Engage with various scenarios to better understand bond energies. Often, standard enthalpies provide more precise and reliable data for specific reactions.
Introduction to Energy Cycles
Energy Cycle: A collection of repeated processes in chemical reactions where energy is transferred and transformed. Essential for understanding energy movement through reactions.
- Importance: Hess's Law is imperative for calculating enthalpy changes in complex processes not measurable directly in a single step.
Key Terms:
- Energy Cycle: A sequence of processes where energy transformation occurs.
- Enthalpy Change: The heat change at constant pressure during a reaction.
Step-by-Step Calculation Guidance for Hess's Law Application
Step 1: Identify the Cycle
- Determine initial and final reaction points to assist in accurate energy mapping.
- Example: Evaluate the pathway from A to B.
Identifying the start and end points is crucial for precise understanding of energy transitions.
Step 2: Break Down Energy Cycle Paths
- Segment the cycle into known reactions, enhancing precision with tabulated enthalpies.
- Sub-note: "Using tabulated values ensures reliable enthalpy outcomes."
Step 3: Apply Hess's Law
- Apply using:
- Emphasise using recalibration by comparing tabulated enthalpies.
Energy Cycle Diagrams
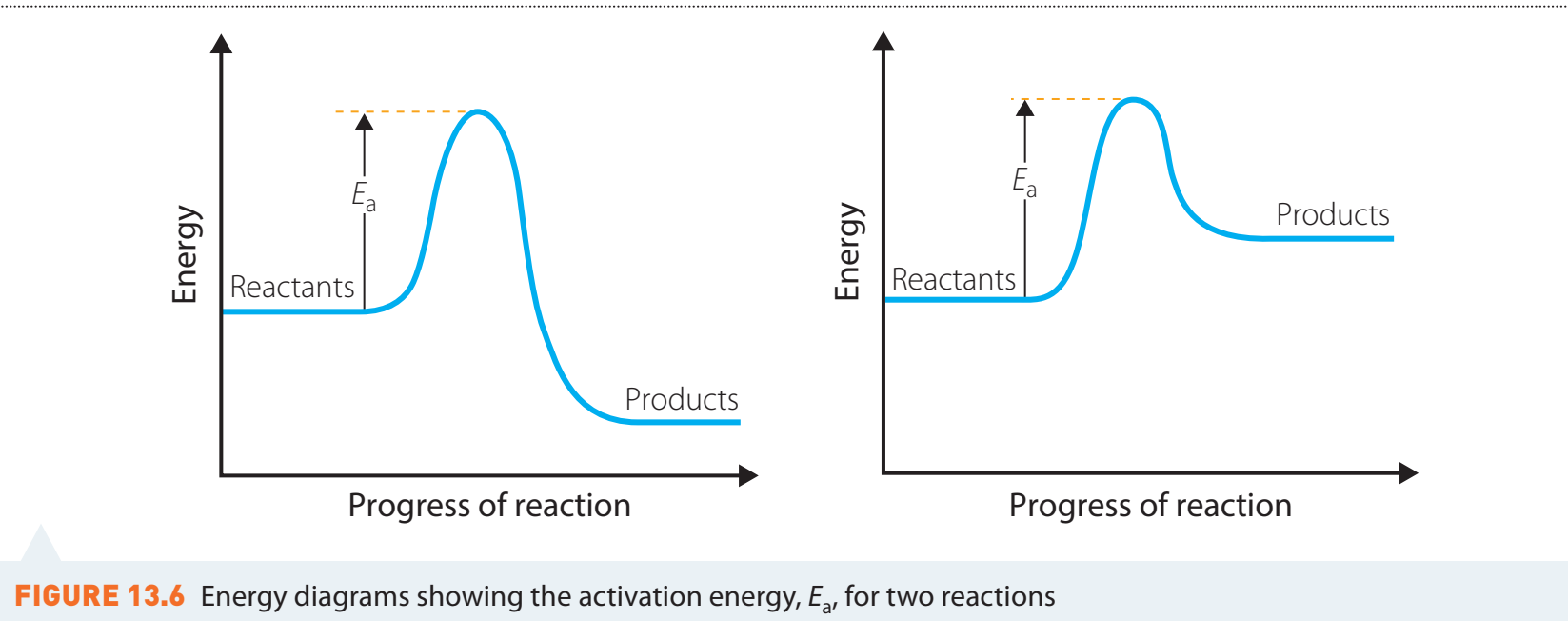
- Guidance: Regularly consult diagrams for better insight into energy transitions.
- Use each diagram appropriately during specific calculations to ensure clarity.
Visualise processes by integrating diagrams with calculations.
Types of Energy Cycles in Chemistry
Combustion Cycles:
- Describes energy release during fuel combustion.
- Applications include evaluating engine efficiencies.
Photosynthesis Cycles:
- Describes energy absorption promoting plant growth.
- Pertains to renewable energy developments.
Respiration Cycles:
- Involves cellular-level energy utilisation.
- Provides necessary energy for cellular functions.
Calculation Example of a Hypothetical Energy Cycle
Follow these steps to calculate enthalpy:
- Divide reaction into smaller steps: A -> B, B -> C.
- Known enthalpy values: .
- Apply Hess's Law:
- Total: .
- Key Insight: Summing known values aids efficient enthalpy calculation.
Industry Application: This technique contributes to energy audits and enhancing efficiencies.
Common Misconceptions and Problem Solving
- Confusing Pathways: Leads to incorrect enthalpy calculations.
- Solutions:
- Confirm calculations with tabulated data.
- Illustrate corrections to ensure reliability.
Summary
- Comprehend Energy Cycles: Clarify roles in energy transformations.
- Employ Hess's Law: Effectively compute total enthalpy changes.
- Leverage Diagrams: Assist in visual learning and problem conceptualisation.
- Maintain consistent study habits and address misconceptions.
Reflect on these principles and techniques to deepen your understanding of energy cycles and the application of Hess's Law. Consider questions such as, How might you apply Hess's Law to a newly encountered energy cycle, and what methods would you utilise?
Introduction to Heat of Combustion
- Heat of Combustion: Enthalpy is a measure of heat energy released or absorbed during a reaction. The heat of combustion represents the enthalpy change when one mole of a substance is completely burned in oxygen.
- Consider a campfire. The heat felt is the release of energy as wood burns.
- Importance: Grasping enthalpy is essential for evaluating energy transformations in chemistry, influencing applications like energy from fuels.
- Examples include power plants and engines.
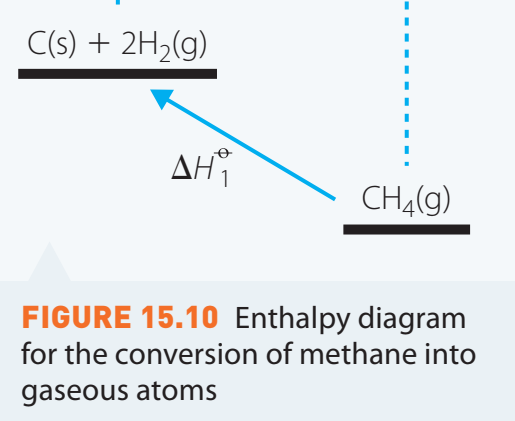
Detailed Case Study: Combustion of Methane
- Significance of Methane: As a major component of natural gas, methane plays a key role in energy generation.
- Chemical Equation:
- Methane combustion reaction:
- Involved Components:
- Methane (CH): Energy source.
- Oxygen (O): Necessary for the reaction's progression.
- Carbon Dioxide (CO) and Water (HO): By-products affecting climate.
- Methane's straightforward structure enables efficient and cleaner burning fuel.
- Methane combustion reaction:
Enthalpy Calculation Approach
- Available Methods:
- Standard Enthalpies of Formation: Used to assess energy change during reactions by examining predefined enthalpies of substances.
- Why Employ Various Methods: Utilise bond energies to corroborate results, especially useful for complex molecules.
Calculation Example - Enthalpy of Combustion
Worked Example
-
Step 1: Identify standard enthalpies for reactants and products.
-
Step 2: Calculate using the formula:
- Sample Calculation:
- CH: kJ/mol, Utilise C from CO and HO: Use provided values.
- Complete Calculation:
- Reactants: CH ( kJ/mol) and O ( kJ/mol)
- Products: CO ( kJ/mol) and HO ( kJ/mol × 2)
- kJ/mol
- Sample Calculation:
-
Step 3: Validate with bond energies. Emphasise the importance of this verification.
-
Comparison Table Format:
Reactant/Product Standard Enthalpy (kJ/mol) Calculated Result Methane (CH) -74.85 Oxygen (O) 0.00 Carbon Dioxide (CO) -393.5 Water (HO) -241.8
Addressing Common Difficulties
- Frequent Errors:
- Misinterpreting table values or inaccurately averaging bond energies.
- Ensuring proper alignment of bonds during calculations.
Verification is Crucial: Always recheck calculations using different methods for accuracy.
Guided Exercise: New Fuel Calculation
-
Overview: Examine an alcohol fuel.
-
Steps:
- Establish the combustion equation for the chosen fuel.
- Compute using standard tables.
- Juxtapose with known data, and account for differences in observations.
-
Global Context: Exploring alternative energy sources underscores scientific advancements in sustainable energy.
Diagrams
Introduction to Photosynthesis
Photosynthesis: An endothermic process where light energy is used to convert carbon dioxide (CO₂) and water (H₂O) into glucose (C₆H₁₂O₆) and oxygen (O₂).
- Photosynthesis Significance:
- Essential for Earth's carbon cycle
- Affects global energy equilibrium
- Sustains autotrophic life, enabling them to produce food from sunlight
1. Standard Equation for Photosynthesis
-
Chemical Equation:
-
Hess's Law Role:
- Hess's Law is used to determine enthalpy change using combustion data.
-
Visual Flowchart:
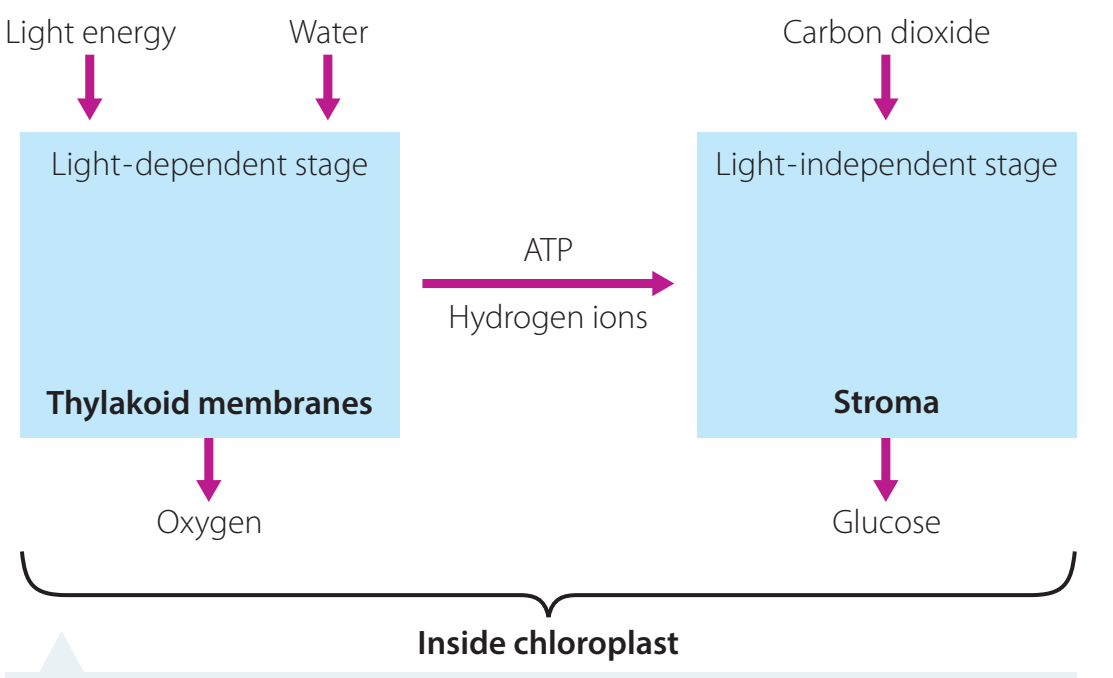
- Illustrates photosynthesis stages with inputs and outputs, assisting comprehension of energy changes.
2. Use of Hess's Law in Photosynthesis
-
Indirect Measurement:
- Direct measurement of enthalpy change is intricate.
- Hess's Law aids in indirect determination using familiar reaction data.
-
Combustion Process:
- Utilises glucose combustion reversal to infer photosynthesis enthalpy.
3. Calculation Examples
Setup: Follow these steps:
- Identify Known Data:
- Use standard enthalpies of formation for glucose and reactants.
- Apply Hess's Law:
- Apply using inverted combustion data.
- Execute Calculations:
- Insert values and compute the total enthalpy change.
- Example Calculation:
Worked Example
- Presume standard enthalpy values are given.
- Insert values into the Hess's Law formula:
- The positive value confirms this is an endothermic process requiring energy input.
4. Factors Impacting Photosynthesis
-
Environmental Impacts:
- Light intensity, temperature, and CO₂ levels significantly influence photosynthesis rates.
-
Species-specific Adaptations:
- Pathways utilised by C3 and C4 plants vary in efficiency.
- Example: C4 plants adapted for high-light environments are more efficient.
-
Diagrams:
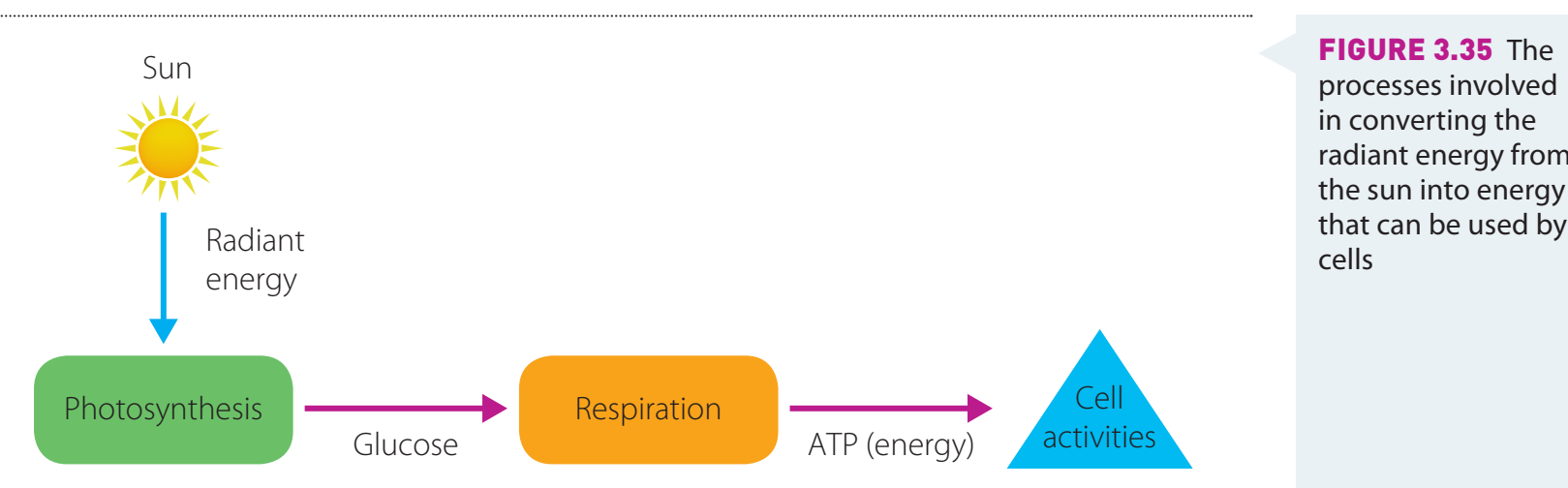
- Outlines energy level variations throughout the process.
Respiration: A Key Biological Energy Transformation
Respiration is the conversion of nutrient energy to ATP, powering cells.
- Process Contrast:
- Photosynthesis: Absorbs light energy, resulting in positive enthalpy changes. Example: Glucose formation.
- Respiration: Releases energy, marked by negative enthalpy changes. Example: Energy release from glucose combustion.
Sequential Phases of Respiration
Glycolysis
- Location: Cytoplasm
- Energy Release: Moderate; critical during high-intensity exercise.
Krebs Cycle
- Connection: Further glucose derivative breakdown.
- Energy Release: Major, significant for thermogenesis.
Oxidative Phosphorylation
- Function: Substantial energy release producing ATP.
- Importance: ATP acts as the primary energy carrier.
Enthalpy Change Calculations
Glucose Combustion Reaction:
- Simplicity of negative enthalpy - confirming energy release.
- Hess's Law Application:
- Simplifies enthalpy evaluations.
- Brief example: Step-by-step energy transformation breakdown.
Correcting Misunderstandings
| Misconception | Fact |
|---|---|
| Perfect energy conservation in biological reactions. | Energy is lost as heat and in other forms. |
| Photosynthesis and respiration are direct inverses. | They complement each other like fitting puzzle pieces. |
Demonstrations and Application
-
Diagrams in Use:
- Visual aids, such as flowcharts, place theory in a practical framework, promoting understanding.
-
Experiment Details:
- Calorimetry provides practical insight into energy measurement.
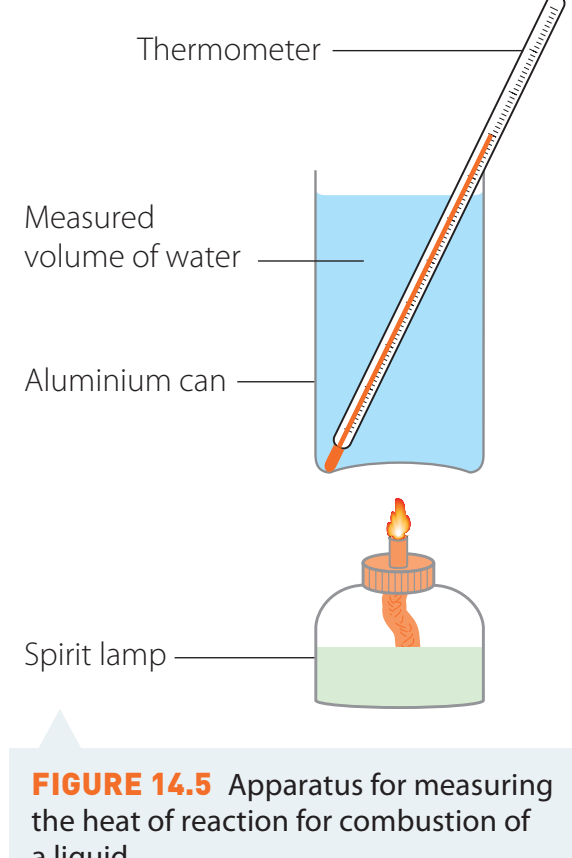
Visual Coordination
- Utilise diagrams like:
500K+ Students Use These Powerful Tools to Master Enthalpy and Hess's Law For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
241 flashcards
Flashcards on Enthalpy and Hess's Law
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards24 quizzes
Quizzes on Enthalpy and Hess's Law
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes16 questions
Exam questions on Enthalpy and Hess's Law
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Enthalpy and Hess's Law
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Enthalpy and Hess's Law
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Enthalpy and Hess's Law you should explore
Discover More Revision Notes Related to Enthalpy and Hess's Law to Deepen Your Understanding and Improve Your Mastery
