Photo AI
Last Updated Sep 24, 2025
Enthalpy - Energy Processes Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Enthalpy - Energy Processes quickly and effectively.
335+ students studying
Enthalpy - Energy Processes
Introduction to Enthalpy
Enthalpy (H): The total heat content of a system, is a fundamental property in thermochemistry.
- Enthalpy assesses the total heat in a system, reflecting its current state. It is crucial for identifying energy changes during chemical reactions.
Enthalpy Change (ΔH): The heat absorbed or released at constant pressure.
- Determines the heat exchange during reactions, helping classify them as either exothermic or endothermic.
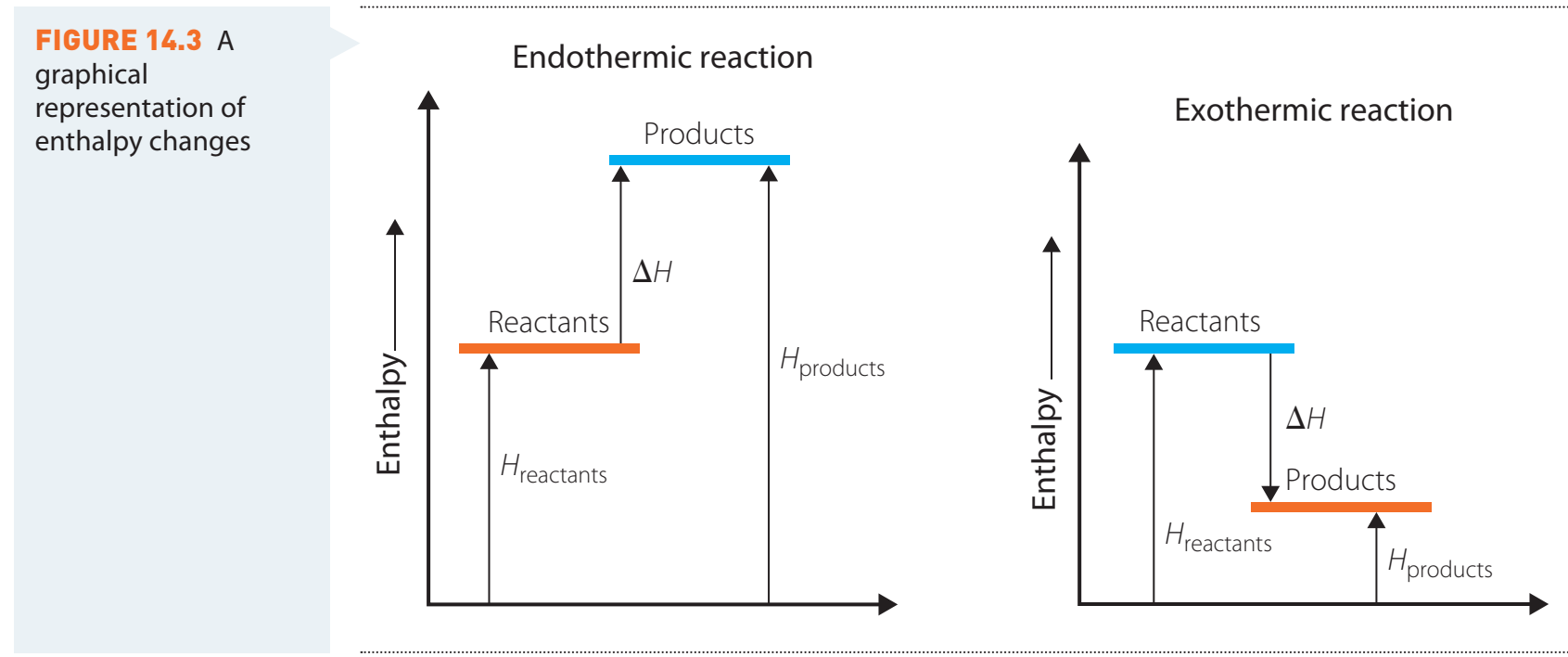
Exothermic vs. Endothermic Reactions
-
Exothermic Reactions:
- Release heat (negative ΔH).
- Example:
-
Endothermic Reactions:
- Absorb heat (positive ΔH).
- Example:
Enthalpy Change Formula
- This is determined by subtracting the enthalpy of reactants from that of the products.
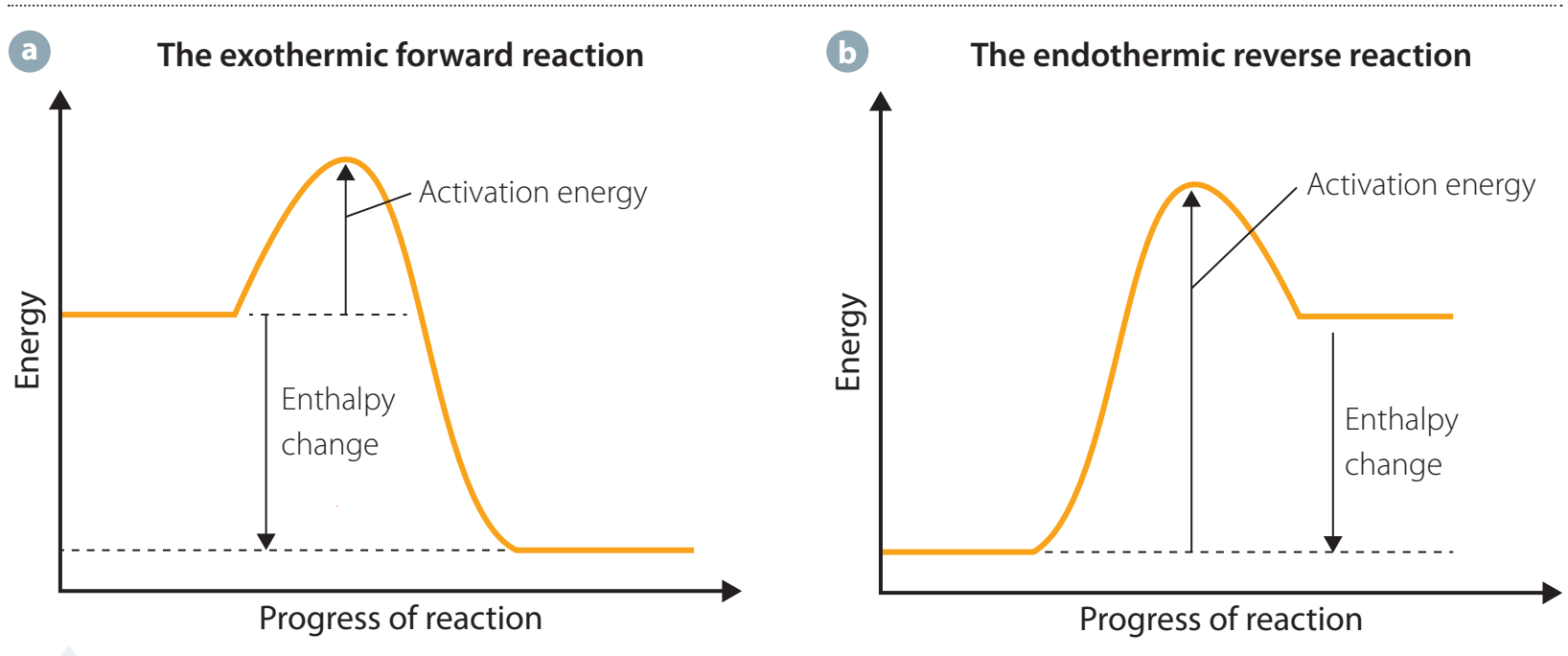
The Application of Hess's Law
Hess's Law: The total change in enthalpy of a chemical reaction is independent of its pathway.
- Utilises known enthalpy changes to estimate energy changes that are challenging to measure directly.
Photosynthesis
- Definition: Photosynthesis is an endothermic process converting carbon dioxide and water into glucose and oxygen.
- It absorbs energy, illustrating its endothermic nature.
Chemical Equation
-
Balanced Reaction:
- ΔH = +2814 kJ/mol.
Stages Involved
Light-dependent Reactions
- Occur in thylakoid membranes, capturing light energy via chlorophyll.
Calvin Cycle
- Takes place in the stroma.
- ATP and NADPH facilitate glucose synthesis.
Factors Influencing ΔH
- The wavelength of light, temperature, and environmental conditions affect the efficiency of photosynthesis.
Respiration
- Definition: Cellular respiration is an exothermic process that releases energy by breaking down glucose.
Chemical Equation
-
Balanced Reaction:
- ΔH = -2814 kJ/mol.
Biochemical Pathways
Glycolysis
- Converts glucose into pyruvate, releasing small amounts of energy.
Krebs Cycle
- Produces NADH and FADH₂.
Oxidative Phosphorylation
- Occurs in mitochondria, generating ATP through the electron transport chain.
Influence of Conditions
- Temperature affects enzyme activity.
- Oxygen levels impact the efficiency of respiration.
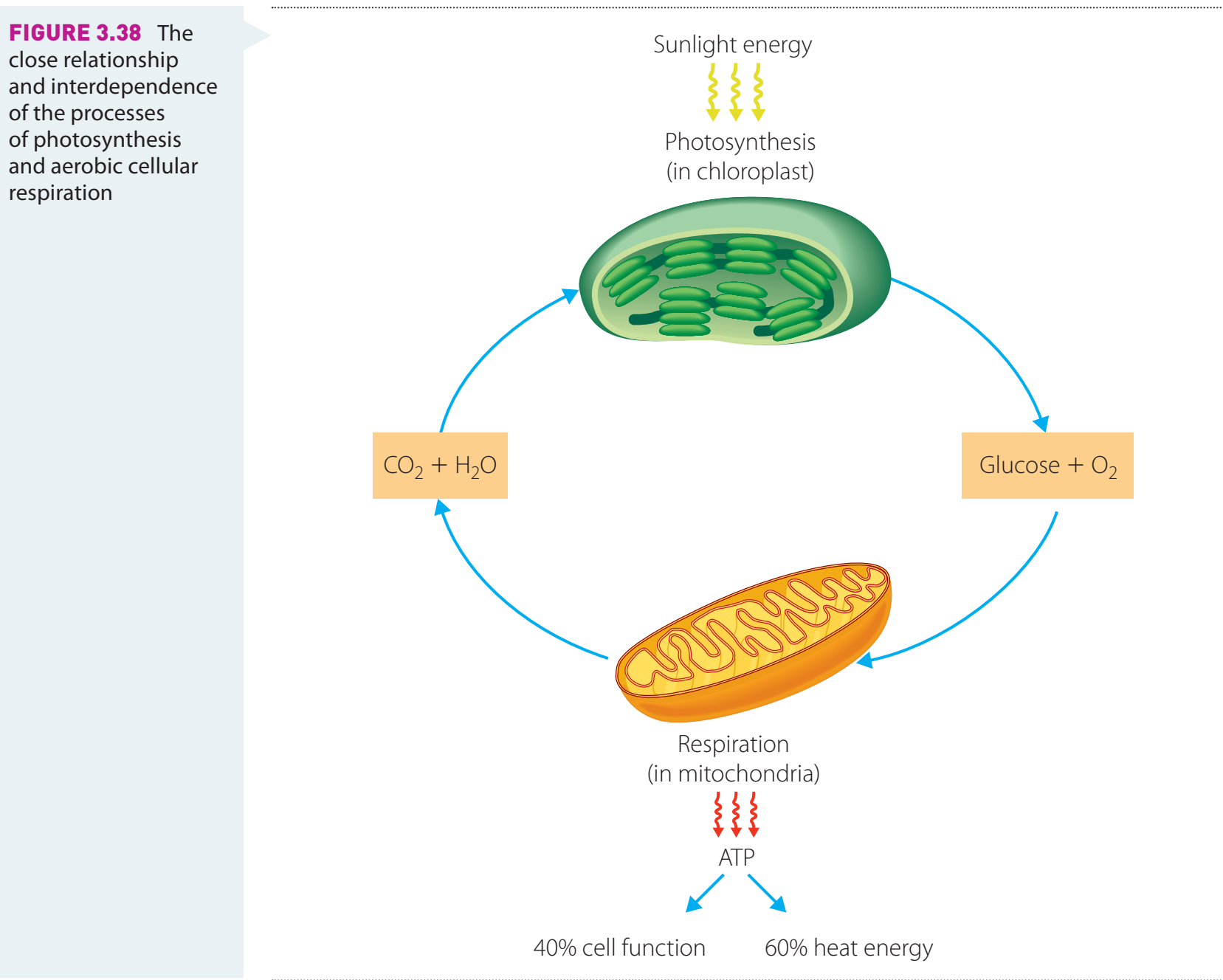
Integrating Hess's Law with Photosynthesis and Respiration
- Photosynthesis stores energy in glucose.
- Respiration releases this stored energy through the breakdown of glucose.
- The Enthalpy Cycle demonstrates the flow and conservation of energy between these processes.
Worked Examples
Example 1: Calculating Enthalpy Change in Photosynthesis
Problem: Calculate the total enthalpy change when 3 moles of glucose are produced during photosynthesis.
Solution:
- The enthalpy change for producing 1 mole of glucose is +2814 kJ/mol
- For 3 moles: ΔH = 3 × (+2814 kJ/mol) = +8442 kJ
Example 2: Using Hess's Law in Respiration
Problem: Calculate the energy released when 2 moles of glucose undergo complete cellular respiration.
Solution:
- The enthalpy change for respiration of 1 mole of glucose is -2814 kJ/mol
- For 2 moles: ΔH = 2 × (-2814 kJ/mol) = -5628 kJ
- Therefore, 5628 kJ of energy is released
Ensure precise calculations of ΔH values for accurate applications in both biochemical and industrial contexts.
Identifying and Mitigating Errors
- Ensure accurate calorimetry measurements and control over environmental conditions.
Precise ΔH measurements are essential for industrial applications and the efficiency of chemical manufacturing.
500K+ Students Use These Powerful Tools to Master Enthalpy - Energy Processes For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
241 flashcards
Flashcards on Enthalpy - Energy Processes
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards24 quizzes
Quizzes on Enthalpy - Energy Processes
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes16 questions
Exam questions on Enthalpy - Energy Processes
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Enthalpy - Energy Processes
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Enthalpy - Energy Processes
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Enthalpy - Energy Processes you should explore
Discover More Revision Notes Related to Enthalpy - Energy Processes to Deepen Your Understanding and Improve Your Mastery
