Photo AI
Last Updated Sep 24, 2025
Enthalpy and Enthalpy Change Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Enthalpy and Enthalpy Change quickly and effectively.
211+ students studying
Enthalpy and Enthalpy Change
Introduction to Enthalpy
What is Enthalpy?
Enthalpy (H): represents the total energy content within a system. It is essential in identifying energy shifts during chemical reactions.
- State Function: Dependent only on the initial and final states of a system, and independent of the path taken.
Standard Enthalpy Change (ΔH)
- Standard Enthalpy Change (ΔH): Refers to the energy change under defined standard conditions:
- Pressure: 1 atm
- Temperature: 298 K
Types of Enthalpy Changes
Enthalpy of Reaction (ΔH₍ᵣₓₙ₎)
- Enthalpy of Reaction (ΔH₍ᵣₓₙ₎): Specifies the energy change occurring during a reaction.
- Example: The release of energy from wood combustion.
Enthalpy of Formation (ΔH₍f₎)
- Enthalpy of Formation (ΔH₍f₎): Energy required to form one mole of a compound from its constituent elements in their elemental states.
Enthalpy of Combustion (ΔH₍c₎)
- Enthalpy of Combustion (ΔH₍c₎): Energy released upon the combustion of one mole of a substance.
- Example:
- Combustion of methane:
Visualising Exothermic and Endothermic Processes
- Exothermic Processes: Release heat with a negative ΔH, such as a campfire.
- Endothermic Processes: Absorb heat with a positive ΔH, such as ice melting.
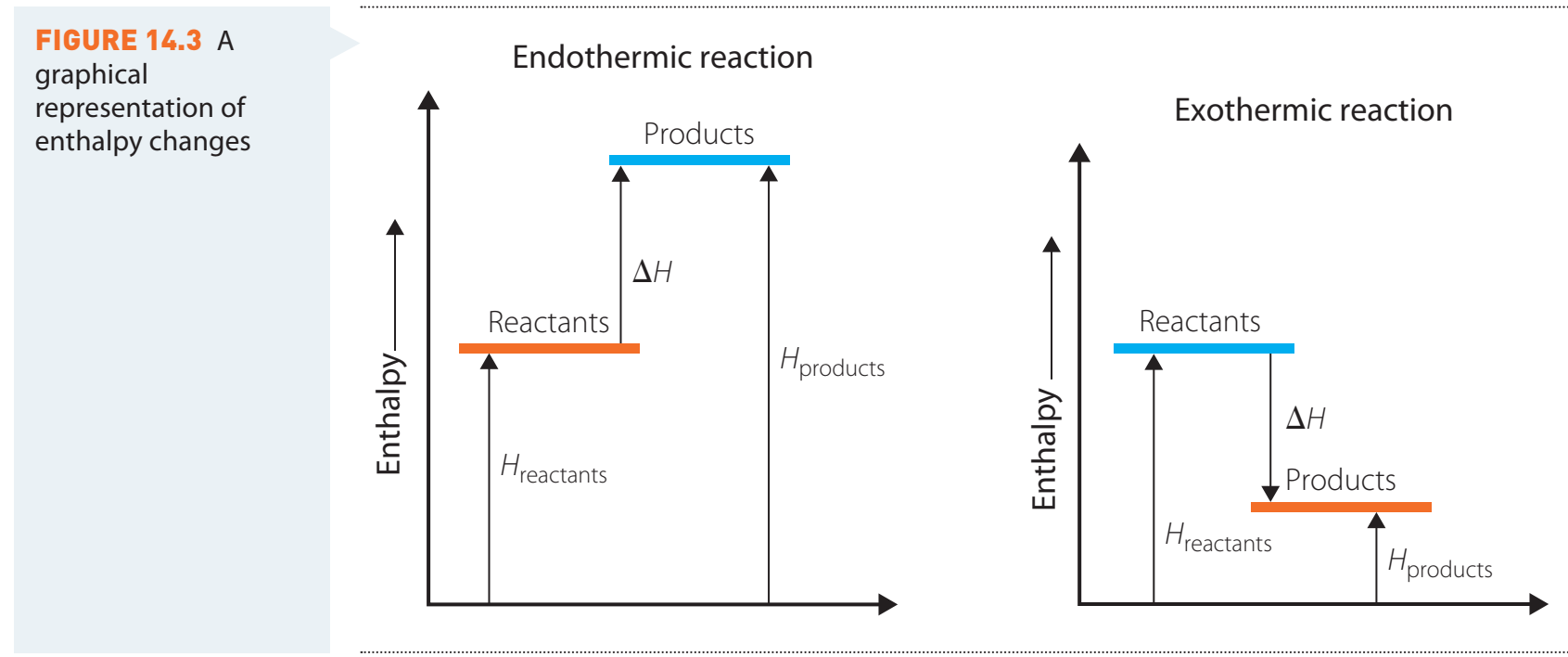
This diagram illustrates the energy flow and heat exchange in different processes.
Connection between Enthalpy and Entropy
- Entropy: Represents the degree of disorder in a system.
- Both are crucial for predicting the spontaneity of reactions using Gibbs Free Energy.
Depicts factors influencing reaction spontaneity.
Understanding enthalpy changes enables the determination of energy absorbed or released in chemical reactions.
Introduction to Calorimetry
- Calorimetry: A method used to measure the heat exchanged during chemical reactions or physical transformations.
Calorimetry: Quantifies heat changes associated with reactions or physical alterations.
Types of Calorimeters
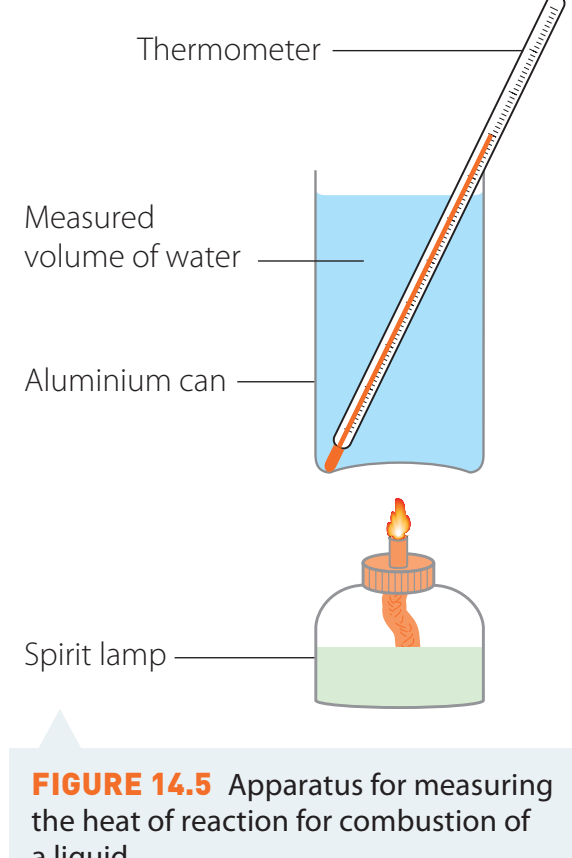
1. Simple Calorimeters
- Structure: An insulated container equipped with a thermometer.
- Usage: Ideal for straightforward educational experiments.
- Disadvantages: Substantial heat loss reduces accuracy.
2. Bomb Calorimeters
- Structure: Insulated, pressure-resistant apparatus.
- Usage: Provides accurate measurements in combustion energy analyses.
- Advantages: Enhanced precision due to reduced heat loss.
Calorimeters are crucial for precise laboratory measurements of energy changes.
Using the Formula q = mCΔT
Importance of the Formula
The q = mCΔT formula is essential for determining heat changes, akin to calculating the energy required to boil water.
Explanation of the Formula
- q: Heat energy change, measured in joules (J).
- m: Mass of the substance, in grams (g) or kilograms (kg).
- C: Specific heat capacity, in J/g°C.
- ΔT: Temperature change.
Definitions:
- q: Change in heat energy.
- m: Mass of the substance.
- C: Specific heat capacity.
- ΔT: Change in temperature.
Sample Calculations
Example 1: Basic
-
Calculate the heat absorbed by 100 g of water, from 25°C to 75°C.
-
Given information:
- Mass (m) = 100 g
- Specific heat capacity of water (C) = 4.18 J/g°C
- Temperature change (ΔT) = 75°C - 25°C = 50°C
-
Solution:
- Using the formula q = mCΔT
- q = 100 g × 4.18 J/g°C × 50°C
- q = 20,900 J or 20.9 kJ
Formula Application:
- Calculation:
Ensure unit consistency; mass should be in grams.
Analysing and Comparing Data
Comparing Experimental Data to Values
- Reliable Sources: Ensure accuracy by referencing academic journals, databases, and textbooks.
- Data Comparison Table: Provides a visual comparison between experimental and literature values.
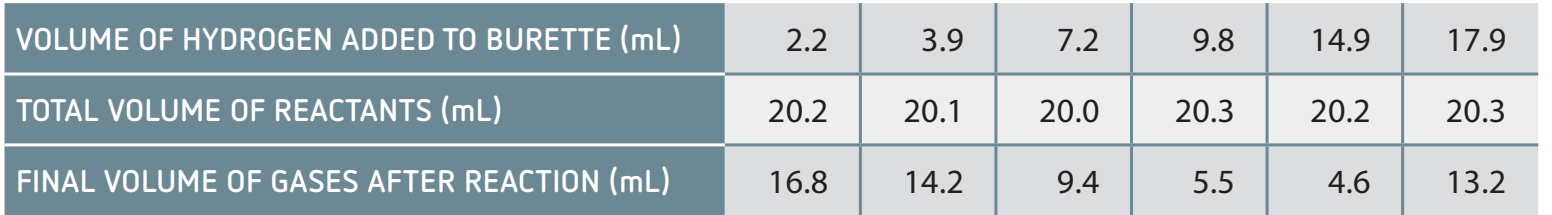
Identifying and Discussing Discrepancies
- Common Discrepancies: Heat loss can affect results, and calibration errors may impact accuracy.
Repeated trials enhance data reliability.
Case Studies and Examples
Exothermic Reaction Case Study
Combustion of Methane
- Formula:
Data Analysis:
- Evaluate heat loss and the completeness of combustion with examples of discrepancies.
Proper insulation minimises heat loss.

Endothermic Reaction Case Study
Dissolution of Ammonium Nitrate
- Formula:
Ensure thorough accounting of all absorbed heat.
Ammonium nitrate is utilised in cold packs.
These notes provide essential insights into understanding enthalpy changes, applications of calorimetry, and data analysis for conducting refined experiments and enhancing concept retention. Accurate interpretation and application facilitate problem-solving in real-world contexts, improving energy management, industrial processes, and academic performance.
500K+ Students Use These Powerful Tools to Master Enthalpy and Enthalpy Change For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
241 flashcards
Flashcards on Enthalpy and Enthalpy Change
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards24 quizzes
Quizzes on Enthalpy and Enthalpy Change
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes16 questions
Exam questions on Enthalpy and Enthalpy Change
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Enthalpy and Enthalpy Change
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Enthalpy and Enthalpy Change
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Enthalpy and Enthalpy Change you should explore
Discover More Revision Notes Related to Enthalpy and Enthalpy Change to Deepen Your Understanding and Improve Your Mastery
