Photo AI
Last Updated Sep 24, 2025
Parts of a Galvanic Cell Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Parts of a Galvanic Cell quickly and effectively.
243+ students studying
Parts of a Galvanic Cell
Introduction
Definition of a Galvanic Cell
- Galvanic Cell (Voltaic Cell): An apparatus that transforms chemical energy into electrical energy through redox reactions.
- Applications:
- Used in electronic devices, such as batteries in laptops and phones.
- Applicable in large-scale energy solutions, including grid systems in power plants.
Practical Insight:
- Galvanic cells are integral to renewable energy solutions, enhancing energy storage and optimisation in solar and wind power systems.
Historical Context
- Alessandro Volta: Invented the first voltaic pile, which was a pivotal development in the conversion of electrical energy.
- Timeline Highlights:
- 1800: Volta develops the voltaic pile.
- Mid-1900s: Emergence of alkaline batteries.
- Today: Innovation in lithium-ion technology fuelling smartphones and electric vehicles.
Environmental Impact: Galvanic cells are vital in reducing reliance on fossil fuels, thus making significant contributions to cleaner energy solutions.
Fundamental Concepts
Redox Reactions
- Reduction: The gain of electrons, crucial for energy storage.
- Oxidation: The loss of electrons, propelling electron flow in cells.
Broader Uses: Beyond simple batteries, redox reactions are essential in corrosion prevention and facilitate integration within renewable energy cycles.
Circuitry Insight
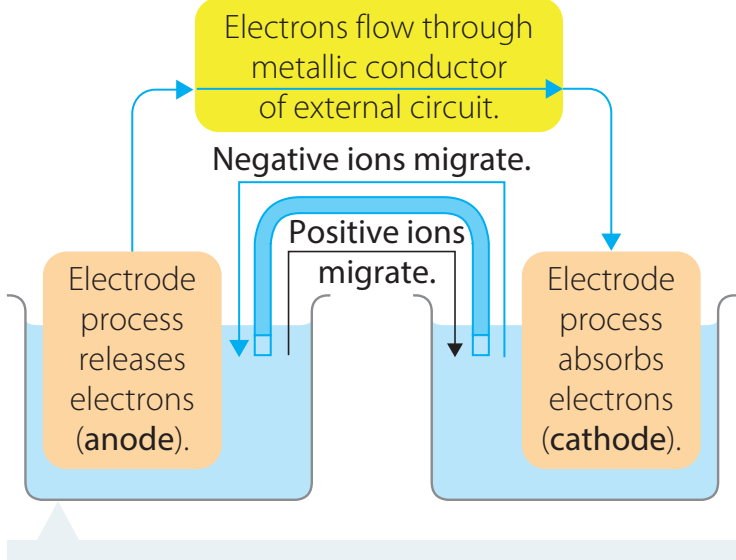
- Electron Flow: Electrons move from the anode to the cathode.
- Potential Difference:
- Drives the movement of electrons through the external circuit.
- Essential for maintaining the functionality of the circuit.
Component Functions and Interactions
Anode Function
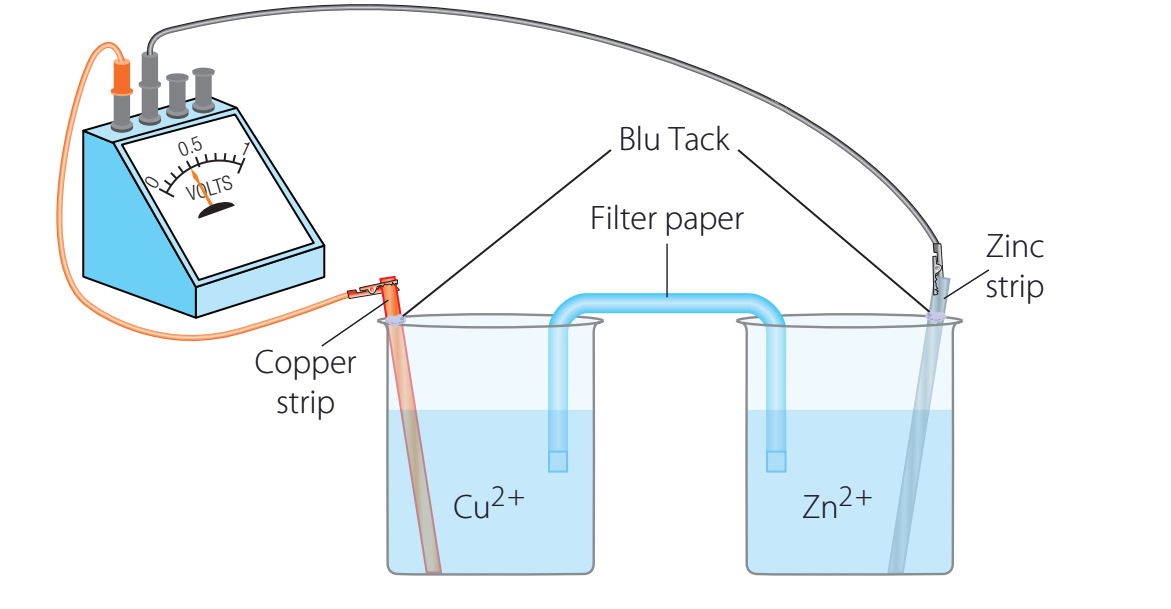
- Anode: The anode serves as the negative terminal where oxidation occurs.
- Materials: Zinc is often used due to its reactivity with oxygen and its high surface area, which promotes oxidation.
- Reaction Example: A standard half-equation is .

Anode: The location of oxidation and the negative terminal in a galvanic cell.
Cathode Function
- Cathode: The cathode operates as the positive terminal where reduction is facilitated.
- Materials: Silver is utilised for its high electrode potential, indicating a more straightforward reduction. The reduction of silver is represented by .
Cathode: Positive terminal where reduction reactions occur, accompanied by electron gain.
Electrolyte Solutions
- Role: These solutions enable ionic conductivity to maintain charge balance.
- Examples: and enhance efficiency by promoting ion movement.
Electrolyte Solutions: Facilitate ion mobility to maintain charge equilibrium.
Salt Bridge
- Function: The salt bridge sustains electrical neutrality by balancing charges between the two half-cells.
- Materials: Commonly used materials, such as , ensure equilibrium. Gel-based innovations can elevate performance.
Salt Bridge: Preserves electrical neutrality by permitting ion flow for charge equilibrium.
External Circuit's Role
- Role: Provides a pathway for electron flow generated from redox reactions at both terminals.
- Efficient Materials: Copper is frequently employed due to its superior conductive properties.

External Circuit: Pathway facilitating electron movement from anode to cathode.
Intercomponent Interactions
An understanding of the interactions among components is crucial for efficient cell operation:
- The anode and cathode initiate electron flow, aided by the external circuit.
- Electrolyte solutions and the salt bridge ensure continuity and balance, vital for ongoing reactions.

A well-functioning galvanic cell depends on seamless interaction among all components to effectively convert energy.
Problem Solving and Key Calculations
- Electron Flow Direction:
- Example: In a zinc-copper cell, zinc atoms at the anode lose electrons (oxidation: ), which then flow through the external circuit to the copper cathode where copper ions gain electrons (reduction: ).
Electrons consistently flow from the anode to the cathode.
- Cell Potential Calculations:
- Example: To calculate the cell potential of a zinc-copper cell:
- Standard reduction potential of copper:
- Standard reduction potential of zinc:
- Cell potential:
- Example: To calculate the cell potential of a zinc-copper cell:
Quick Revision Key Points
- Anode: Site of oxidation.
- Cathode: Location of reduction.
- Electrolyte: Facilitates ionic movement and circuit completion.
- Salt Bridge: Maintains charge balance and neutrality.
- External Circuit: Enables electron transfer.
Mnemonic: "LEO the lion says GER" for: Loss of Electrons is Oxidation, Gain of Electrons is Reduction.
Exam Tips
- Flow Direction: Confirm electron flow in diagrams is from anode to cathode.
- Component Roles: Clearly identify components with accurate labels in exams.
- Fun Fact/Did You Know?: Redox reactions are fundamental to battery function, including those in electric cars.
Self-Check Q&A:
- What direction do electrons flow in a galvanic cell?
- Solution: Electrons flow from the anode (negative terminal) to the cathode (positive terminal) through the external circuit.
- Describe the roles of the anode and cathode in these reactions.
- Solution: The anode is where oxidation occurs (loss of electrons), while the cathode is where reduction occurs (gain of electrons).
- How do galvanic cells aid in renewable energy?
- Solution: Galvanic cells provide energy storage solutions for intermittent renewable sources like solar and wind, enabling energy to be stored when generated and used when needed.
Exam Tip: Focus on comprehending the role of each part and their interdependence. Note that typical exam questions often involve analysing these interactions.
500K+ Students Use These Powerful Tools to Master Parts of a Galvanic Cell For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
144 flashcards
Flashcards on Parts of a Galvanic Cell
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards22 quizzes
Quizzes on Parts of a Galvanic Cell
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes21 questions
Exam questions on Parts of a Galvanic Cell
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Parts of a Galvanic Cell
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Parts of a Galvanic Cell
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Parts of a Galvanic Cell you should explore
Discover More Revision Notes Related to Parts of a Galvanic Cell to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Galvanic Cells and Standard Electrode Potentials
Galvanic Cells and Potentials
312+ studying
199KViews96%
114 rated
Galvanic Cells and Standard Electrode Potentials
Galvanic Cells
248+ studying
198KViews96%
114 rated
Galvanic Cells and Standard Electrode Potentials
Galvanic Cells and Potentials
324+ studying
185KViews96%
114 rated
Galvanic Cells and Standard Electrode Potentials
Galvanic Cells
466+ studying
194KViews