Photo AI
Last Updated Sep 24, 2025
Galvanic Cells Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Galvanic Cells quickly and effectively.
208+ students studying
Galvanic Cells
This section explores galvanic cells, their variations, functions, and importance in electrochemistry. These cells utilise spontaneous redox reactions to generate electrical energy, which is pivotal for applications such as batteries.
Definition and Function
-
Galvanic Cell: An electrochemical device that employs a spontaneous redox reaction to produce electrical power.
-
Purpose: Transforms chemical energy into electrical energy.
- Applied in practical devices such as batteries and other electronics.
- Provides power to everyday items such as remote controls and torches.
Galvanic cells spontaneously generate electricity, which is indispensable for various applications.
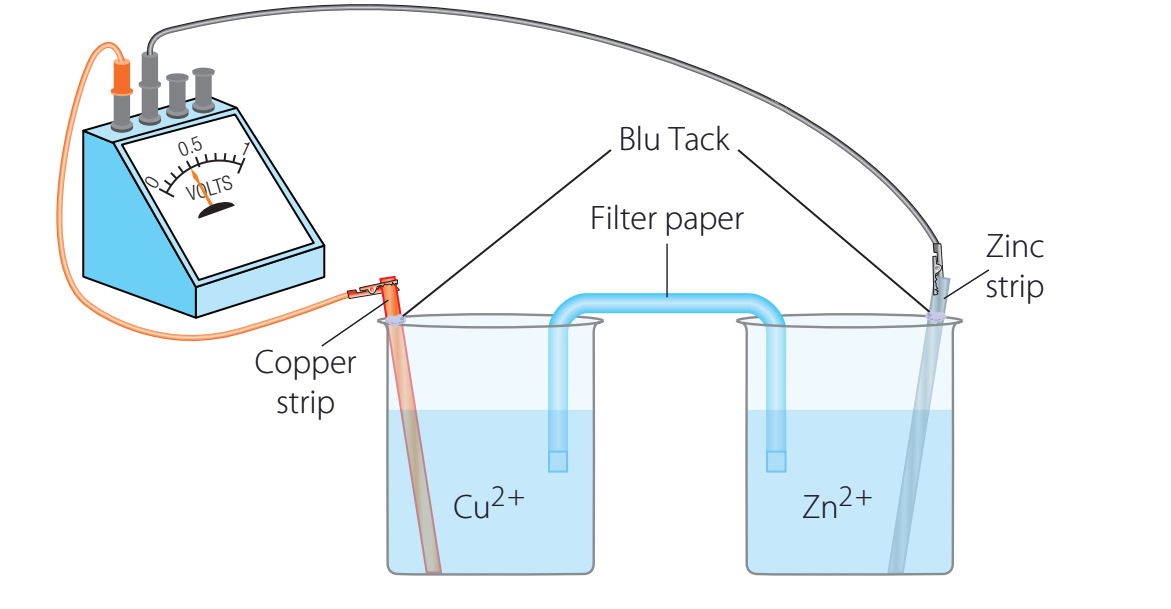
Components of a Galvanic Cell
-
Anode:
- Serves as the site of oxidation.
- Electrons are liberated and directed towards the cathode.
-
Cathode:
- Functions as the site of reduction.
- Electrons are accepted at this location.
-
Salt Bridge:
- Preserves electrical neutrality in the system.
- Prevents charge buildup, facilitating uninterrupted operation.
-
Electrolytes:
- Allow ion movement between electrodes.
- Support ion flow effectively.
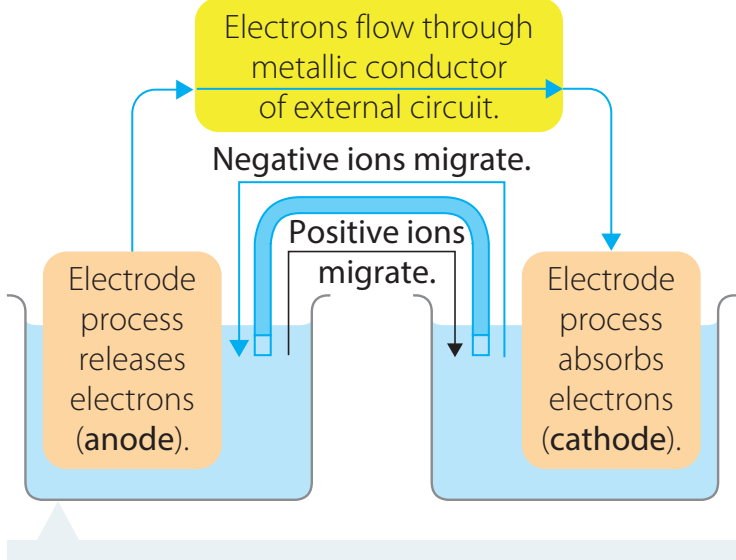
Working Principle
-
Electron Flow:
- Electrons travel from anode to cathode, generating current due to the potential difference.
-
Ion Migration:
- Ions transfer through the solution and the salt bridge.
- Ensures stability by promoting uniform ion movement.
-
Redox Reactions:
- Oxidation: Defined by the loss of electrons.
- Reduction: Characterised by the gain of electrons.
Zinc-Copper Reaction
Setup & Importance: The oxidation of zinc is akin to lighting a match, leaving electrons as residue. In contrast, copper's reduction is comparable to a sponge, absorbing these electrons to sustain continuous flow, thus generating electricity for practical uses.
Electrons emitted at the anode are pivotal in driving the circuit, essential for device functionality.
-
Anode Reaction:
-
Cathode Reaction:
Historical Significance
-
Development:
- Pioneers such as Luigi Galvani and Alessandro Volta made significant contributions.
- Their early experiments formed the basis of galvanic cell theory.
-
Daniell Cell:
- The first reliable galvanic cell, marking advancement in electrochemical engineering.
The historical development of galvanic cells has highlighted important technological strides.
Visual Component
Concentration Cells
- Definition: Concentration Cell: A form of galvanic cell using the same components at differing concentrations.
- Function: Electrical current stems from concentration gradients, akin to water levels balancing between two tanks.
Construction and Function
- Setup:
- Comprise identical electrodes immersed in solutions of varying concentrations.
- Segregated by a semipermeable membrane.
The concentration gradient fundamentally impacts the cell's voltage and efficiency.
Nernst Equation
-
Equation:
-
Example Calculation:
- For a concentration cell with nickel electrodes in 0.1 M and 1 M solutions:
- Using the Nernst equation where , , , and
Fuel Cells
- Definition: Fuel Cells transform chemical energy directly to electrical energy with a continuous provision of reactants.
- Eco-Friendly: Lowers carbon emissions due to negligible pollutants.
Chemical Reactions
- Anode:
- Cathode:
Applications
- Automotive: Hydrogen fuel cell vehicles offer a sustainable transport solution.
- Power Generation: Provide a reliable and portable power source.
Dry Cells
- Definition: Dry Cells: Electrochemical cells lacking free-flowing liquid electrolytes.
Chemical Reactions
-
Anode (Oxidation):
-
Cathode (Reduction):
-
Use: Suited for portable devices like remote controls and torches.
Comparison
| Feature | Zinc-Carbon | Alkaline |
|---|---|---|
| Cost | Lower | Higher |
| Energy Density | Limited | Greater |
Reversibility in Batteries
- Definition: Reversibility: In rechargeables, reversing chemical reactions restores initial reactants.
Lead-Acid Battery
- Reactions:
- Anode: Transformation from lead sulphate to lead.
- Cathode: Lead oxide reforms from lead sulphate.
Lithium-Ion Battery
- Process:
- Charging: Lithium ions accumulate energy.
- Discharging: Ions release stored energy.
Environmental and Economic Considerations
-
Environmental:
- Reduction in waste when using rechargeables instead of disposables.
-
Economic:
- Although initially costlier, rechargeables offer long-term savings.

500K+ Students Use These Powerful Tools to Master Galvanic Cells For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
144 flashcards
Flashcards on Galvanic Cells
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards22 quizzes
Quizzes on Galvanic Cells
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes21 questions
Exam questions on Galvanic Cells
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Galvanic Cells
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Galvanic Cells
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Galvanic Cells you should explore
Discover More Revision Notes Related to Galvanic Cells to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Galvanic Cells and Standard Electrode Potentials
Galvanic Cells and Potentials
211+ studying
200KViews96%
114 rated
Galvanic Cells and Standard Electrode Potentials
Parts of a Galvanic Cell
401+ studying
183KViews96%
114 rated
Galvanic Cells and Standard Electrode Potentials
Galvanic Cells and Potentials
325+ studying
182KViews96%
114 rated
Galvanic Cells and Standard Electrode Potentials
Galvanic Cells
417+ studying
191KViews