Photo AI
Last Updated Sep 24, 2025
The Mole Concept Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand The Mole Concept quickly and effectively.
349+ students studying
The Mole Concept
Introduction to the Mole Concept
Overview
- The mole is a key aspect of chemistry.
- It serves as a link between minuscule atomic particles and macroscopic quantities.
- Enables comprehension of tiny entities in a manageable way.
Historical Context
- Developed for measuring and quantifying atoms and molecules.
- Amedeo Avogadro's contributions were pivotal in formulating the mole.
- Avogadro's Hypothesis: Crucial for progress in modern chemistry.
Significance in Chemistry
- Essential for chemical calculations and understanding molecular quantities.
- Interdisciplinary applications:
- Physics: Mass computations in nuclear processes.
- Biology: Measurement of concentrations in solutions.
- Pharmacy: Accurate dosage formulations.
- Example: Calculating flour in baking parallels using moles in substances.
Key Terminology
- Mole: A counting unit, like a dozen, contains entities.
- Example: 1 mole of carbon equals carbon atoms.
Mole: Enables micro-scale counting, fundamental in chemistry.
- Avogadro's Constant: The number of entities per mole .
- Example: 1 mole of water equals water molecules.
Avogadro's Constant: Links atomic-level understanding to quantifiable amounts.
Relevance to Students
- Understanding moles is crucial for mastering chemical equations and computations.
- Important for practical applications in labs and advanced chemistry courses.
- Common Misunderstanding: Avoid confusing a mole with a basic unit like "one"; it equates to approximately entities.
Engaging Exploration
- Interactive Question: "If a mole of candy was compared to a dozen, how large would each appear?"
- Challenge: Imagine sweets versus merely 12.
Grasping moles in calculations parallels using precise measurements in recipes – comprehend both principles and methods.
Highlights
- Utilising moles in laboratories is comparable to following recipes, establishing a basis for empirical formulas and resolving complex issues.
Diagrams
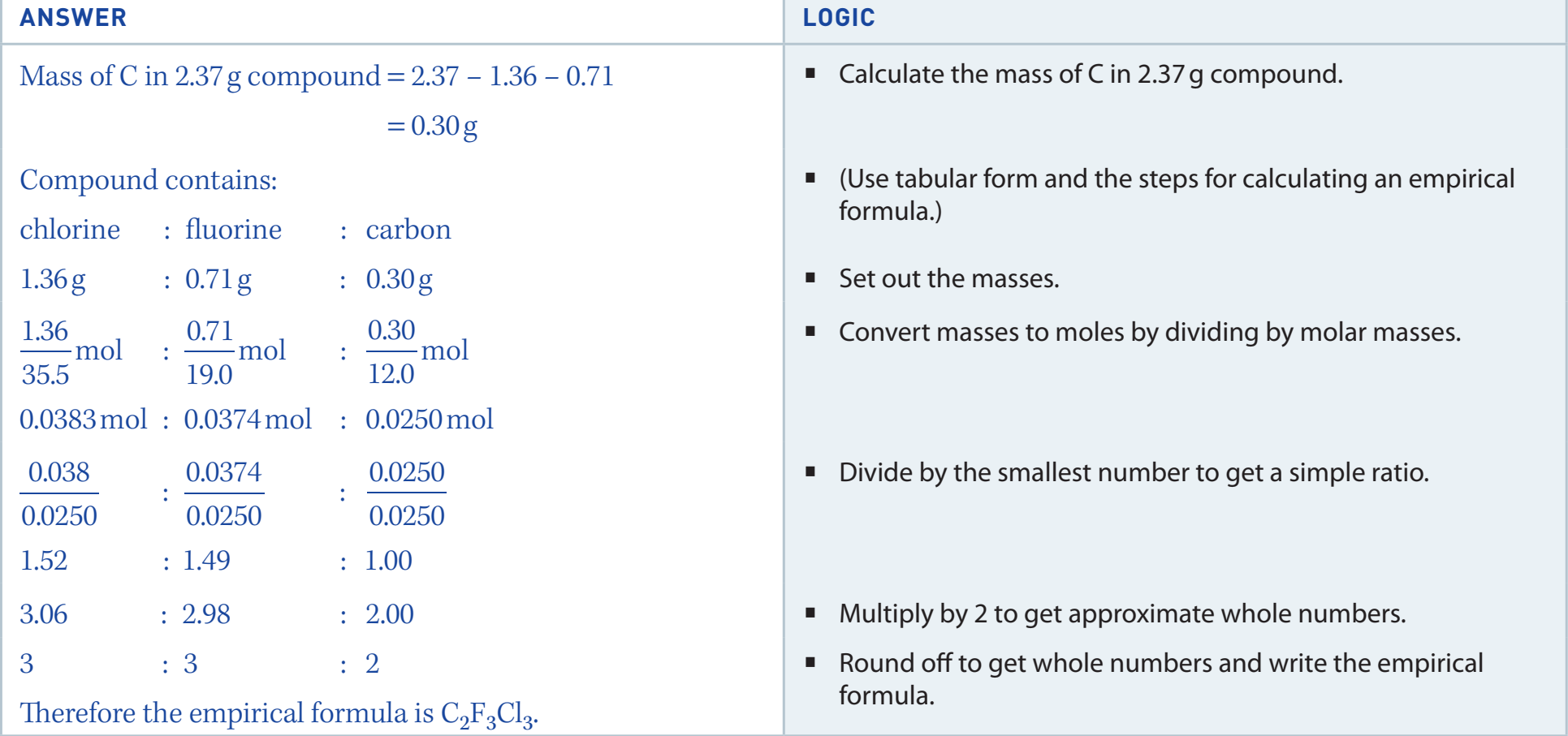
Definition of Empirical Formula
- Empirical Formula: Represents the simplest integer ratio of elements in a compound.
- Difference from Molecular Formula: The empirical formula reveals the simplest ratio, whereas the molecular formula shows the precise number of atoms in a molecule.
- Example: Glucose, molecular formula C₆H₁₂O₆, has an empirical formula of CH₂O.
Empirical Formula: Simplest integer ratio, vital in chemical analysis.
Definition and Significance
-
Percentage Composition by Mass: Indicates the weight percent of each element in a compound.
-
Significance:
- Empirical Formulas: Key for deriving the simplest element ratio in a compound.
- Material Properties: Essential in evaluating material performance under various conditions.
- Chemical Analysis: Widely applied in assessing compound content and purity.
Real-World Importance: Percentage composition is pivotal in quality assurance in chemical manufacturing and providing nutritional information.
Calculation Method
Step-by-Step Procedure
-
Identify Chemical Formula:
- Example: For Iron(III) Oxide, the formula is Fe₂O₃.
-
Calculate Molar Mass:
- Formula Box:
- Fe = 55.85 g/mol, O = 16.00 g/mol
- Total molar mass of Fe₂O₃ = g/mol
- Formula Box:
-
Calculate Mass Contribution:
- Iron in Fe₂O₃:
- Percentage =
- Oxygen in Fe₂O₃:
- Percentage =
- Iron in Fe₂O₃:
Common Errors: Ensure accuracy with molar masses and avoid computational mistakes.
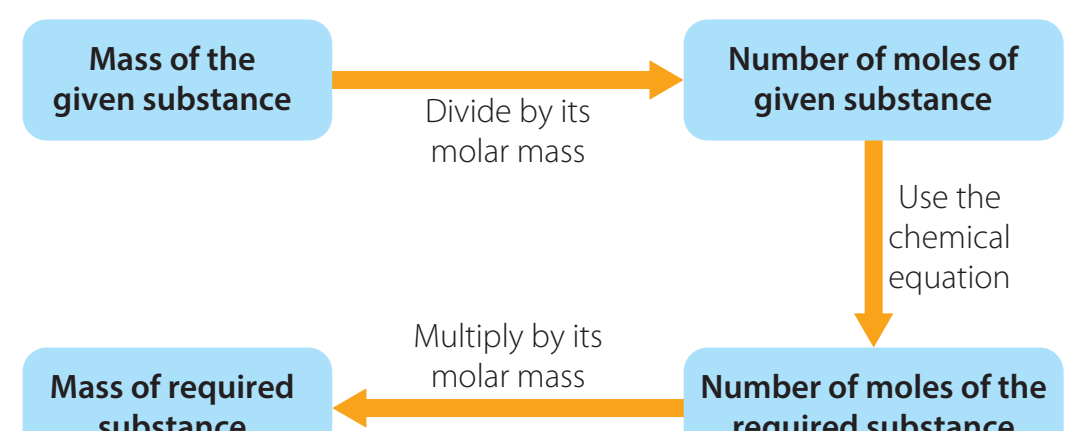
Overall Process Overview and Step-by-Step Calculation
Step 1: Convert Percentage to Mass
- Assume a hypothetical 100 g sample for straightforward conversion.
Step 2: Convert Mass to Moles Using Molar Mass
- Refer to the periodic table to ascertain molar masses for each element.
- Tip: Precise molar masses prevent computation mistakes.
Step 3: Divide by the Smallest Number of Moles
- Divide each mole quantity by the smallest mole value to achieve a straightforward ratio.
Step 4: Convert Ratios to Whole Numbers
- Translate the remaining mole ratios into whole numbers.
Worked Examples
Example 1: Magnesium Oxide
- Composition: 60.3% magnesium, 39.7% oxygen
- Solution:
- Convert to grams: In a 100g sample, we have 60.3g Mg and 39.7g O
- Convert to moles:
- Moles of Mg = 60.3g ÷ 24.3g/mol = 2.48 mol
- Moles of O = 39.7g ÷ 16.0g/mol = 2.48 mol
- Find ratio: Mg = 2.48:2.48 = 1:1
- Empirical formula: MgO
Example 2: Water
- Composition: 11.19% hydrogen, 88.81% oxygen
- Solution:
- Convert to grams: In a 100g sample, we have 11.19g H and 88.81g O
- Convert to moles:
- Moles of H = 11.19g ÷ 1.01g/mol = 11.08 mol
- Moles of O = 88.81g ÷ 16.0g/mol = 5.55 mol
- Find ratio: H = 11.08:5.55 = 2:1
- Empirical formula: H₂O
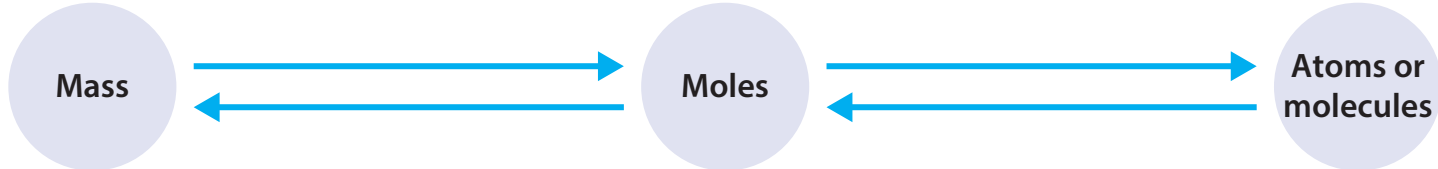
Connecting Moles to Chemical Equations
Chemical Equation Balancing
Chemical Equation Balancing: Ensures the conservation of mass by keeping equations balanced.
- Balancing ensures equal atom quantities for each element on both sides.
Steps for Balancing Equations
Common Mistakes:
- Do not alter subscripts; modify coefficients only.
Quick Tips
- Balance one element at a time.
- Count atoms to guarantee they are equal on both sides.
Explanation of Avogadro's Constant in Real-World Contexts
- Gas Volume at STP: At Standard Temperature and Pressure (STP), Avogadro's constant indicates that one mole of any gas occupies 22.4 litres.
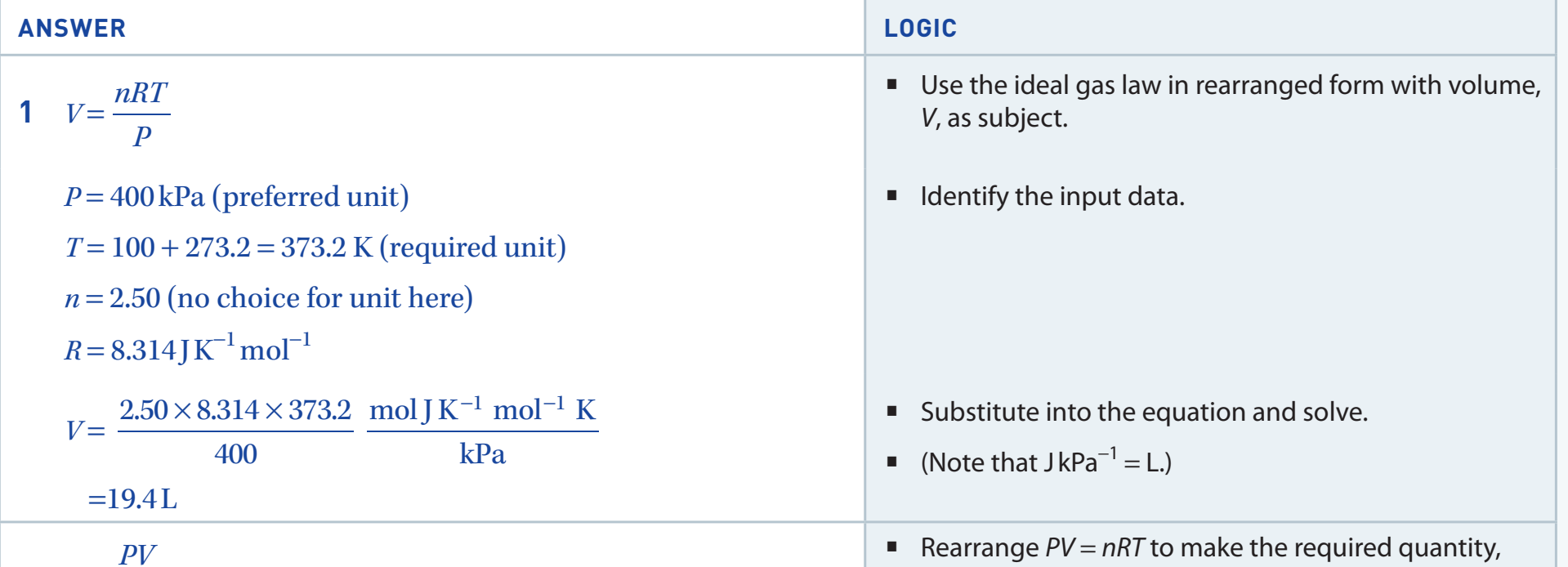
Bridging Micro and Macro Perspectives
- Translates molecules into measurable mass amounts.
1 mole of gas litres at STP particles.
Common Misconceptions
- Moles vs Molecules: Mistakes often arise from equating moles to single molecules, leading to errors in calculations.
500K+ Students Use These Powerful Tools to Master The Mole Concept For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
267 flashcards
Flashcards on The Mole Concept
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards26 quizzes
Quizzes on The Mole Concept
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes7 questions
Exam questions on The Mole Concept
Boost your confidence with real exam questions.
Try Chemistry Questions2 exams created
Exam Builder on The Mole Concept
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on The Mole Concept
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to The Mole Concept you should explore
Discover More Revision Notes Related to The Mole Concept to Deepen Your Understanding and Improve Your Mastery