Photo AI
Last Updated Sep 24, 2025
Organic Acid-Base Reactions Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Organic Acid-Base Reactions quickly and effectively.
475+ students studying
Organic Acid-Base Reactions
Contextual Introduction
Understanding acid-base reactions plays a crucial role in organic chemistry, forming the basis of numerous biochemical processes and laboratory techniques. For Year 12 students, proficiency in this area is essential for curriculum success and assessments.
Definitions
- Bronsted-Lowry Acids and Bases: Within this framework, acids are defined as proton donors, while bases function as proton acceptors. Example: Hydrochloric acid (HCl) donates a proton, transforming into a chloride ion (Cl⁻).
- Lewis Acids and Bases: This concept describes acids as electron pair acceptors and bases as electron pair donors. Example: Boron trifluoride (BF₃) acts as a Lewis acid.
Key Terms
- Proton Donor: A substance that donates a proton (H⁺).
- Electron Pair Acceptor: A substance that accepts an electron pair.
Types and Structures of Organic Acids and Bases
-
Organic Acids: Defined by the presence of a carboxyl group (-COOH), which influences their acidity. Example: Acetic acid (CH₃COOH).
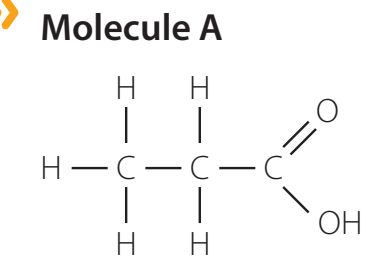
-
Organic Bases: Includes amines, such as ethylamine, characterised by an amino group (-NH₂), affecting their basicity. Example: Ethylamine (C₂H₅NH₂).

Understand how functional groups like -COOH and -NH₂ influence acid/base behaviour.
Diagrams and Examples
Ionisation of Carboxylic Acids
- Carboxylic acids dissociate in water to form carboxylate ions and hydronium ions, demonstrating proton transfer.
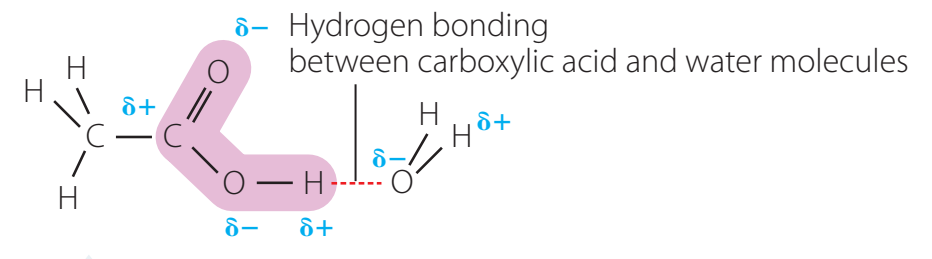
- Example: Formic acid (HCOOH) ionises into formate ions (HCOO⁻) and hydronium ions (H₃O⁺).
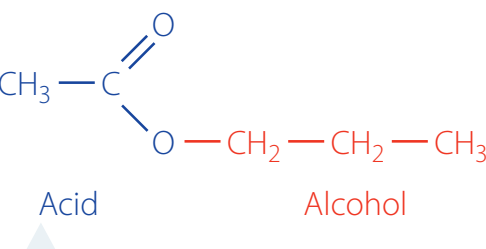
Esterification, Neutralisation, and Hydrolysis
-
Esterification: The process of forming esters from carboxylic acids and alcohols in the presence of acid catalysts, such as sulphuric acid. Removing water drives the reaction towards ester formation.
- Equation:
- Example: Ethanoic acid reacts with ethanol to produce ethyl ethanoate.
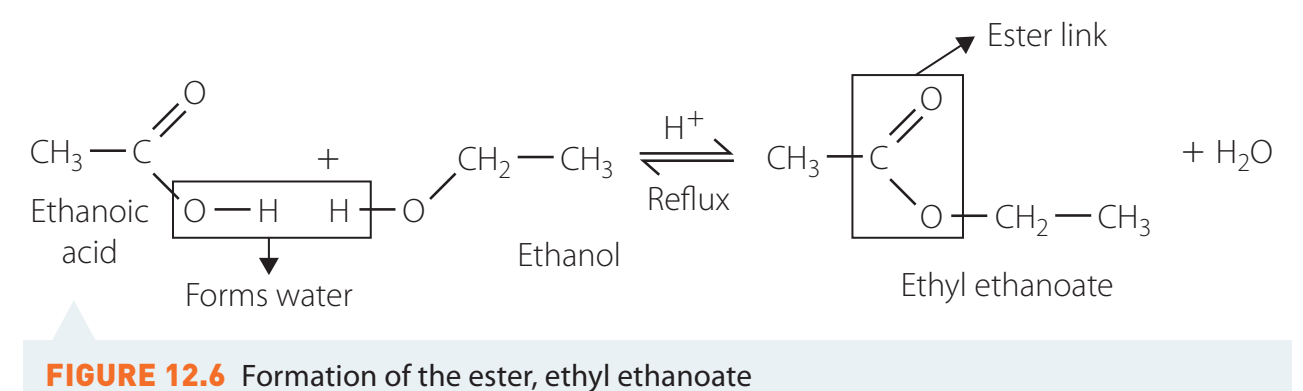
chatImportantApplying Le Chatelier's principle, minimising water promotes ester formation.
-
Acid-Base Neutralisation: This reaction forms salts and water. Example:
-
Hydrolysis: The reverse process of esterification where esters break down into acids and alcohols in the presence of water.
- Equation:
- Example: Methyl butanoate decomposes into butanoic acid and methanol.
Reaction Conditions
-
Temperature and Catalysts:
- Esterification: Requires acid catalysts and heat.
- Hydrolysis: More effective under acidic or basic conditions.
-
Reaction Environment:
- Hydrolysis necessitates water for ester breakdown.
Reaction Pathways and Retrosynthesis
Definition of Reaction Pathways
- Reaction Pathways: These are sequential processes that convert reactants to products in organic synthesis, highlighting their stepwise nature.
Quick Definition: Reaction pathways entail sequences of steps that transform reactants into products in organic synthesis.
Retrosynthesis Principles
-
Retrosynthesis: Deconstructs complex molecules into simpler, constructible parts. This approach is indispensable for planning efficient syntheses.
chatImportantRetrosynthesis Defined: A strategic method for breaking down complex chemical structures to facilitate synthesis planning.
Examples of Reaction Pathways
Ethanol to Ethyl Acetate Conversion
- Step 1: Formation of ethanol.
- Step 2: Oxymercuration leads to an intermediate.
- Step 3: Reacting with acetic acid yields ethyl acetate.
Worked Example:
- Start with ethanol (CH₃CH₂OH)
- Add acetic acid (CH₃COOH) with concentrated H₂SO₄ catalyst
- Heat the mixture to promote the reaction
- The reaction produces ethyl acetate (CH₃COOCH₂CH₃) and water
Intermediates play a critical role in successful transformation.
Propene to Acetone Conversion
- Step 1: Propene undergoes oxidation to form an intermediate.
- Step 2: Intermediate reactions lead to acetone, underscoring intermediates and conditions vital for product formation.
Worked Example:
- Start with propene (CH₃CH=CH₂)
- Add KMnO₄ for oxidation to produce propane-1,2-diol
- Cleave the diol with periodic acid (HIO₄)
- This produces acetone (CH₃COCH₃) and formaldehyde (HCHO)
Introduction to Flow Charts
Flow charts break down complex synthesis processes into simplified, manageable steps essential for planning.
Flow charts should emphasise clarity and precision.
Steps for Creating Flow Charts
1. Identify Starting Materials and Goals
- Specify reactants and desired final product.
- Example: Alcohol to Ester - Ethanol to ethyl acetate.
2. Mapping the Reaction Pathway
- Decompose reactions into smaller, understandable steps.
3. Incorporating Reaction Conditions and Intermediates
- Note temperature, pH, and intermediates using bullet points.
Symbol Usage and Notation
- Arrows: Indicate the direction of reactions.
- Boxes: Highlight important steps.
- Circles: Denote intermediates.
Common Misconceptions and Pitfalls
Typical Pitfalls
- Omits Intermediates: Leads to unclear understanding.
- Incorrect Labelling or Conditions: Causes misunderstandings.
Cross-reference flow charts with established examples for accuracy.
Common Errors in Reaction Equations
-
Incorrect Balancing:
-
Accurate balancing ensures mass conservation.
-
Example: Adjust 'Fe + O₂ → Fe₂O₃' to '4Fe + 3O₂ → 2Fe₂O₃'.
-
Incorrect Reaction Conditions:
-
Verify correctness for temperature and catalysts.
Troubleshooting Strategies
- Inclusion of All Intermediates: Ensure none are missing.
- Symbol Consistency: Maintain uniform use throughout.
- Verification of Balanced Equations: Confirm balance and condition accuracy.
Exam Tip
Develop a strong understanding of mechanisms and processes such as proton and electron transfers to enhance practical skills.
500K+ Students Use These Powerful Tools to Master Organic Acid-Base Reactions For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
138 flashcards
Flashcards on Organic Acid-Base Reactions
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards17 quizzes
Quizzes on Organic Acid-Base Reactions
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes14 questions
Exam questions on Organic Acid-Base Reactions
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Organic Acid-Base Reactions
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Organic Acid-Base Reactions
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Organic Acid-Base Reactions you should explore
Discover More Revision Notes Related to Organic Acid-Base Reactions to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Reactions of Organic Acids and Bases
Organic Acids and Bases
209+ studying
189KViews96%
114 rated
Reactions of Organic Acids and Bases
Organic Acids and Bases
482+ studying
191KViews