Photo AI
Last Updated Sep 24, 2025
Soaps and Detergents Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Soaps and Detergents quickly and effectively.
437+ students studying
Soaps and Detergents
This revision note provides an overview of the reactions of organic acids and bases, emphasising the formation and use of soaps and detergents.
Definitions and Characteristics
- Organic Acids and Bases: Compounds predominantly made of carbon-based structures, particularly characterised by carbon-hydrogen bonds.
Key Characteristics:
- Carboxyl (-COOH) Group: Found in acids such as acetic acid (CH₃COOH), commonly present in vinegar, and lactic acid, which plays a vital role in muscle metabolism.
- Amino (-NH₂) Group: Present in bases like methylamine (CH₃NH₂), utilised in pharmaceuticals. Another instance includes trimethylamine, responsible for the odour in fish.
Acid-Base Reactions
-
Mechanism of Reaction:
- Proton Donation and Acceptance: Fundamental processes that define the behaviours of acids and bases.
- Example reaction: Neutralisation of acetic acid with sodium hydroxide (NaOH):
-
Ka and pKa Values: Represent the strength of an acid, essential in fields such as pharmacology for drug design.
Understanding proton dynamics is crucial for mastering organic reaction mechanisms.
Saponification Process: Making Soap
-
Saponification: This process involves triglycerides reacting with NaOH to produce glycerol and soap:
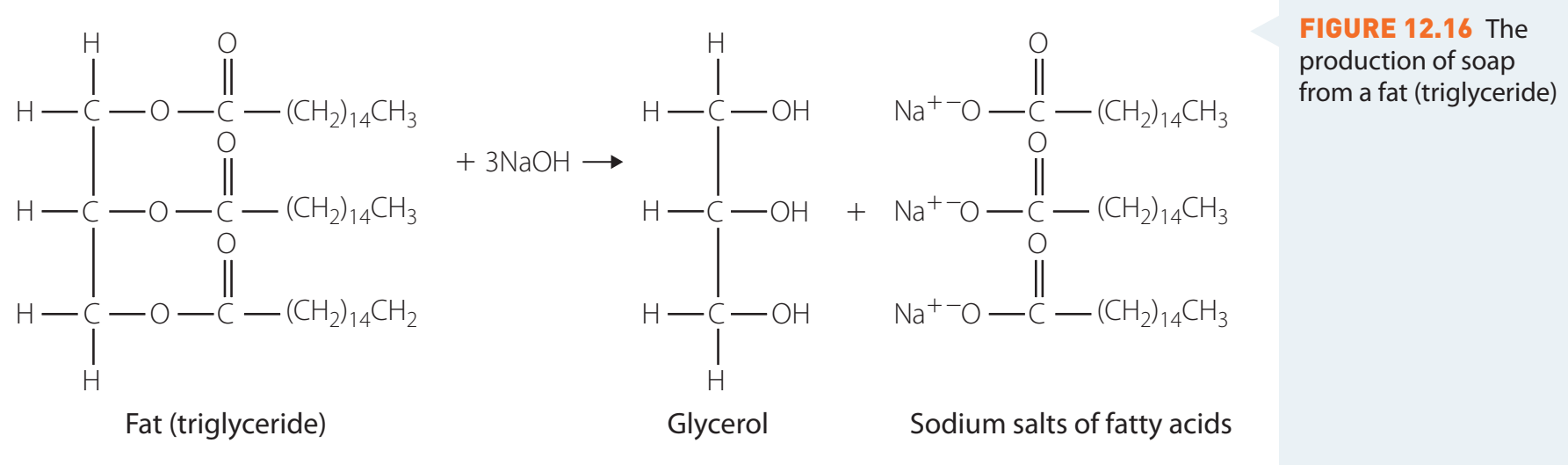
-
Soap Molecules: Structure and Function
- Amphipathic Nature:
- Hydrophilic Head: Attracts water.
- Hydrophobic Tail: Attracts grease.
- Amphipathic Nature:
Detergents: An Alternative to Soaps
- Detergents are synthetic alternatives to traditional soaps, addressing limitations such as reduced effectiveness in hard water.
Chemical Structure
- Structural Differences: Includes sulfonate or sulfate groups, which enhance water solubility and cleaning efficiency.
Analogy: Sulfonate and sulfate groups function like magnets, attracting dirt and moisture.
Types and Uses
- Anionic Detergents:
- Example: Sodium lauryl sulfate, used in shampoos.
- Cationic Detergents:
- Example: Cetyl trimethylammonium chloride, used in fabric softeners.
- Non-ionic Detergents:
- Example: Fatty alcohol ethoxylates, used in dishwashing to control foam.
Environmental Impact
- Synthetic detergents degrade more slowly than natural soaps, leading to environmental concerns like eutrophication. Regulations aim to mitigate these issues through phosphate restrictions and the promotion of biodegradable ingredients.
Regulation focus: Essential for reducing environmental damage and promoting eco-friendly detergent technologies.
Comparative Analysis: Soaps vs. Detergents
Chemical and Physical Properties
- Soap Structures: Comprised of natural organic salts with ionic heads and non-polar tails, derived from natural fats and oils.
- Detergent Structures: Composed with sulfate/sulfonate groups, offering effectiveness across different water types.
| Component Type | Soap | Detergent |
|---|---|---|
| Structure | Organic salt | Sulfate/Sulfonate group |
Solubility
- Hard Water:
- Soaps form scum in the presence of calcium ions, reducing their effectiveness.
- Detergents remain effective, preventing the formation of scum.
Biodegradability and Environmental Concerns
- Soaps: Naturally biodegradable and decompose quickly.
- Detergents: Contain synthetic polymers that persist, impacting aquatic life.
Safety Considerations
- Appropriate PPE is required to avoid incidents such as chemical burns.
Worked Example: Saponification Reaction
Let's explore the saponification reaction in detail:
When a triglyceride reacts with sodium hydroxide:
- The ester bonds in the triglyceride are broken
- Each fatty acid chain forms a sodium salt (soap molecule)
- Glycerol is produced as a by-product
Calculation example: If 1 mole of a triglyceride (molecular weight 890 g/mol) reacts completely with sodium hydroxide:
- 3 moles of NaOH are required (40 g/mol × 3 = 120g)
- 1 mole of glycerol is produced (92 g/mol)
- 3 moles of soap molecules are formed (the remainder of the mass)
Therefore, from 890g of triglyceride, we obtain 92g of glycerol and 918g of soap (890 + 120 - 92 = 918g).
500K+ Students Use These Powerful Tools to Master Soaps and Detergents For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
138 flashcards
Flashcards on Soaps and Detergents
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards17 quizzes
Quizzes on Soaps and Detergents
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes14 questions
Exam questions on Soaps and Detergents
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Soaps and Detergents
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Soaps and Detergents
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Soaps and Detergents you should explore
Discover More Revision Notes Related to Soaps and Detergents to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Reactions of Organic Acids and Bases
Organic Acids and Bases
326+ studying
191KViews96%
114 rated
Reactions of Organic Acids and Bases
Organic Acids and Bases
259+ studying
181KViews96%
114 rated
Reactions of Organic Acids and Bases
Organic Acid-Base Reactions
312+ studying
189KViews