Photo AI
Last Updated Sep 13, 2025
The Glycosidic Bond Simplified Revision Notes for A-Level AQA Biology
Revision notes with simplified explanations to understand The Glycosidic Bond quickly and effectively.
213+ students studying
1.1.5 The Glycosidic Bond
A glycosidic bond is a type of strong covalent bond that forms between two monosaccharides (simple sugars), allowing them to join and form disaccharides (two sugars) or polysaccharides (long chains of sugars). This bond is created when two hydroxyl (-OH) groups from different sugar molecules interact, leading to the removal of a water molecule in a condensation reaction.
-
For example, in maltose, two glucose molecules join together to form a glycosidic bond, resulting in a disaccharide.
-
In sucrose, a glycosidic bond forms between α-glucose and β-fructose to create another disaccharide.
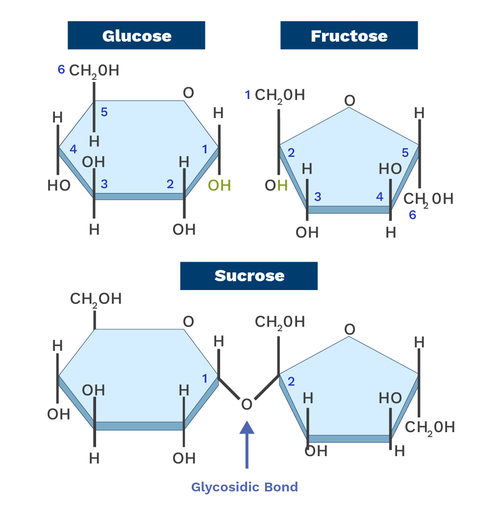
-
In polysaccharides like amylopectin, many glycosidic bonds connect glucose molecules, forming long, branched chains. Glycosidic bonds make sugar molecules more suitable for transport, storage, and they help reduce the osmolarity of cells, preventing water balance issues.
Breaking the Glycosidic Bond
The glycosidic bond can be broken through a process called hydrolysis, where a water molecule is added to split the bond. This reaction breaks down larger sugar molecules, such as disaccharides and polysaccharides, back into their individual monosaccharides.
- Hydrolysis is important in processes like digestion, where large carbohydrates are broken down into smaller sugars that can be absorbed by the body. For example, the breakdown of sucrose into glucose and fructose is an example of hydrolysis. Enzymes are required to catalyse both condensation and hydrolysis reactions.
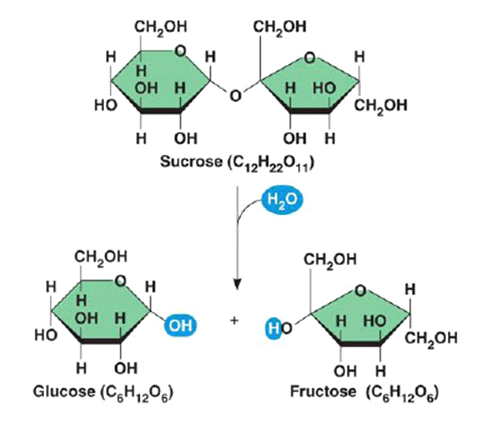
Enzymes and Bond Types
Different types of monosaccharides form different types of glycosidic bonds, such as:
- Maltose has an α-1,4 glycosidic bond.
- Sucrose has an α-1,2 glycosidic bond. These bonds play an essential role in the structure and function of carbohydrates in biological systems, including energy storage (e.g., glycogen, starch) and structural components (e.g., cellulose).
500K+ Students Use These Powerful Tools to Master The Glycosidic Bond For their A-Level Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
110 flashcards
Flashcards on The Glycosidic Bond
Revise key concepts with interactive flashcards.
Try Biology Flashcards11 quizzes
Quizzes on The Glycosidic Bond
Test your knowledge with fun and engaging quizzes.
Try Biology Quizzes3 questions
Exam questions on The Glycosidic Bond
Boost your confidence with real exam questions.
Try Biology Questions9 exams created
Exam Builder on The Glycosidic Bond
Create custom exams across topics for better practice!
Try Biology exam builder17 papers
Past Papers on The Glycosidic Bond
Practice past papers to reinforce exam experience.
Try Biology Past PapersOther Revision Notes related to The Glycosidic Bond you should explore
Discover More Revision Notes Related to The Glycosidic Bond to Deepen Your Understanding and Improve Your Mastery
