Photo AI
Last Updated Sep 27, 2025
Types of Forces Between Molecules Simplified Revision Notes for A-Level AQA Chemistry
Revision notes with simplified explanations to understand Types of Forces Between Molecules quickly and effectively.
480+ students studying
1.5.3 Types of Forces Between Molecules
1. Induced Dipole–Dipole (Van der Waals / London Dispersion) Forces
Induced dipole–dipole forces, also known as van der Waals or London dispersion forces, are the weakest type of intermolecular force. They occur in all atoms and molecules, whether polar or non-polar, due to temporary shifts in the electron distribution.
Formation
- Electrons are constantly moving in their clouds, and at any given moment, they may be unevenly distributed. This causes a temporary dipole in one molecule.
- This dipole can induce a dipole in a neighbouring molecule, leading to an attraction between the two molecules.
- The process can continue, with the induced dipole in one molecule creating dipoles in additional nearby molecules.
Factors Influencing Strength
- The larger the molecule (higher ), the more electrons it has, and the stronger the van der Waals forces.
- For example, larger molecules like iodine () have stronger dispersion forces compared to smaller ones like hydrogen ().
Examples: These forces are significant in non-polar substances like methane () or hexane (), where van der Waals forces are the only type of intermolecular attraction.
2. Permanent Dipole–Dipole Forces
Permanent dipole–dipole forces occur between polar molecules where there is a permanent separation of charge (dipole).
Formation
- In a polar molecule, one end is slightly positive () and the other is slightly negative () due to differences in electronegativity between atoms.
- The end of one molecule attracts the end of a neighbouring molecule, leading to electrostatic attraction between the molecules.
Comparison with Van der Waals Forces
- While polar molecules also experience van der Waals forces, the permanent dipole–dipole forces add an extra layer of attraction, making the overall intermolecular forces stronger in polar substances.
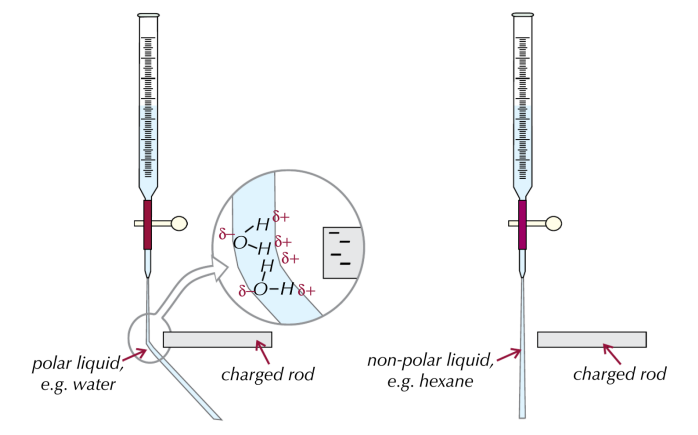
Testing Polar Liquids
You can observe dipole–dipole forces by using an electrostatically charged rod near a jet of a polar liquid, such as water. The polar molecules will be attracted to the rod, causing the liquid to deflect. Non-polar liquids like hexane will not show any deflection.
3. Hydrogen Bonding
Hydrogen bonding is a special type of dipole–dipole interaction and is significantly stronger than both van der Waals and permanent dipole–dipole forces. It occurs when a hydrogen atom is covalently bonded to a very electronegative atom (, , or ) and interacts with the lone pair of another electronegative atom in a different molecule.
Formation
- In molecules like water () or ammonia (), the hydrogen atom bonded to oxygen () or nitrogen () has a partial positive charge (), while the electronegative atom (, , or ) holds a partial negative charge ().
- This highly polar bond, combined with hydrogen's small size and high charge density, results in a strong attraction between the hydrogen atom and a lone pair on a neighboring , , or atom.
Effect on Physical Properties
- Hydrogen bonding is responsible for the unusual properties of substances like water, including its lower density in solid form (ice) and anomalously high boiling points.
Examples:
- Water: The bond in water creates strong hydrogen bonds between water molecules, leading to its high boiling point relative to its molecular weight.
- Ammonia () also exhibits hydrogen bonding, though not as strongly as water.
Summary of Intermolecular Forces
| Type of Force | Strength | Example Substances | Key Feature |
|---|---|---|---|
| Induced Dipole–Dipole | Weak | Methane () and Iodine () | Present in all molecules; increases with size. |
| Permanent Dipole–Dipole | Moderate | Hydrogen chloride () | Attraction between permanent dipoles. |
| Hydrogen Bonding | Strong | Water () and Ammonia () | Involves H bonded to O, N, or F. |
These intermolecular forces significantly influence the melting and boiling points of substances. Stronger forces require more energy to overcome, resulting in higher melting or boiling points.
500K+ Students Use These Powerful Tools to Master Types of Forces Between Molecules For their A-Level Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
40 flashcards
Flashcards on Types of Forces Between Molecules
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards4 quizzes
Quizzes on Types of Forces Between Molecules
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes29 questions
Exam questions on Types of Forces Between Molecules
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Types of Forces Between Molecules
Create custom exams across topics for better practice!
Try Chemistry exam builder21 papers
Past Papers on Types of Forces Between Molecules
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Types of Forces Between Molecules you should explore
Discover More Revision Notes Related to Types of Forces Between Molecules to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Molecules: Shapes & Forces
Shapes of Simple Molecules & Ions
500+ studying
185KViews96%
114 rated
Molecules: Shapes & Forces
Effects of Forces Between Molecules
465+ studying
181KViews