Photo AI
Last Updated Sep 27, 2025
Shapes of Simple Molecules & Ions Simplified Revision Notes for A-Level AQA Chemistry
Revision notes with simplified explanations to understand Shapes of Simple Molecules & Ions quickly and effectively.
317+ students studying
1.5.1 Shapes of Simple Molecules & Ions
Key Principles
Electron Pairs as Charge Clouds
- Bonding pairs and lone pairs (non-bonding) are charge clouds that repel each other.
- Electrons in the outer shell of atoms arrange themselves as far apart as possible to minimize repulsion.
Different Types of Repulsion
- Lone pair-lone pair repulsion is stronger than lone pair-bond pair repulsion.
- Lone pair-bond pair repulsion is stronger than bond pair-bond pair repulsion.
- These differences in repulsion affect bond angles and molecular shapes.
Effect on Bond Angles
- The bond angle decreases as the number of lone pairs increases due to their stronger repulsive forces.
The Valence Shell Electron Pair Repulsion (VSEPR) Theory
- This theory is used to predict the shape of molecules based on the idea that electron pairs around a central atom repel each other.
- Molecules take the shape that allows the electron pairs to be as far apart as possible, minimizing repulsion.
Common Molecular Shapes and Bond Angles
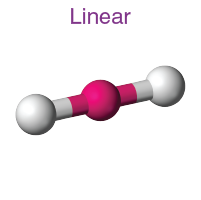
1. Linear Shape
- Example:
- Electron Pairs: 2 bonding pairs, 0 lone pairs.
- Bond Angle: 180°.
- Explanation: The two bonding pairs repel each other, positioning themselves on opposite sides.
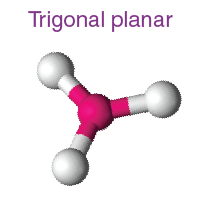
2. Trigonal Planar
- Example:
- Electron Pairs: 3 bonding pairs, 0 lone pairs.
- Bond Angle: 120°.
- Explanation: The bonding pairs are equally spaced around the central atom.
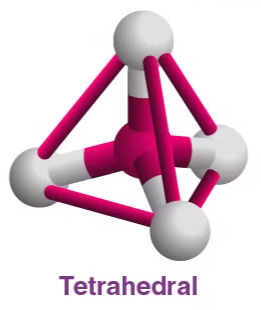
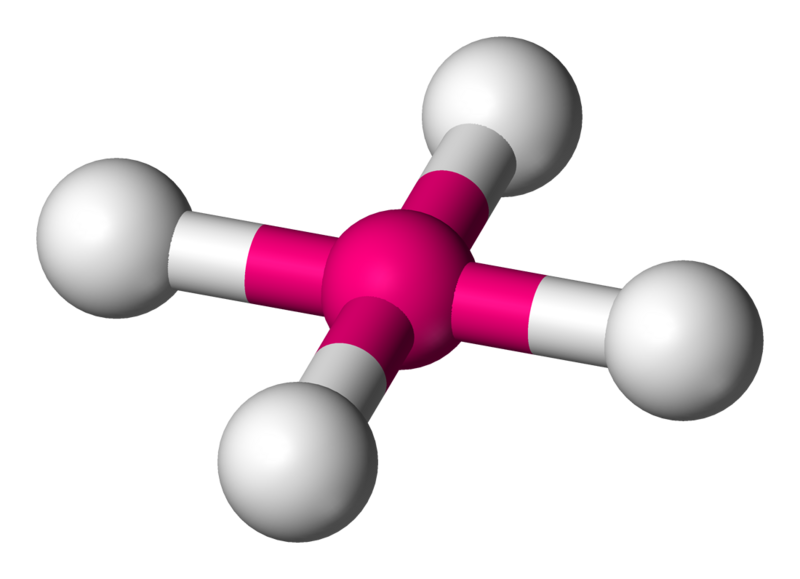
3. Tetrahedral
- Example:
- Electron Pairs: 4 bonding pairs, 0 lone pairs.
- Bond Angle: 109.5°.
- Explanation: The bonding pairs are arranged in a tetrahedral shape to minimize repulsion.
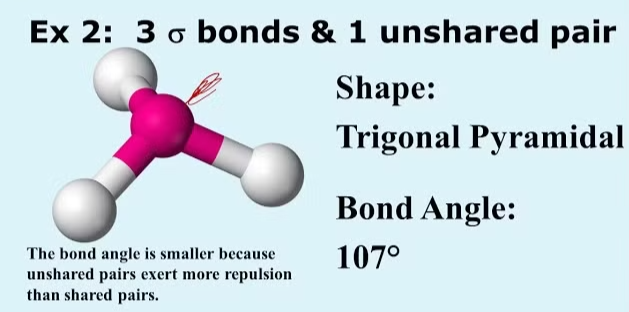
4. Trigonal Pyramidal
- Example:
- Electron Pairs: 3 bonding pairs, 1 lone pair.
- Bond Angle: 107°.
- Explanation: The lone pair repels more strongly, pushing the bonding pairs closer together.
5. Bent or V-Shaped
- Example:
- Electron Pairs: 2 bonding pairs, 2 lone pairs.
- Bond Angle: 104.5°.
- Explanation: Two lone pairs exert stronger repulsion, reducing the bond angle.
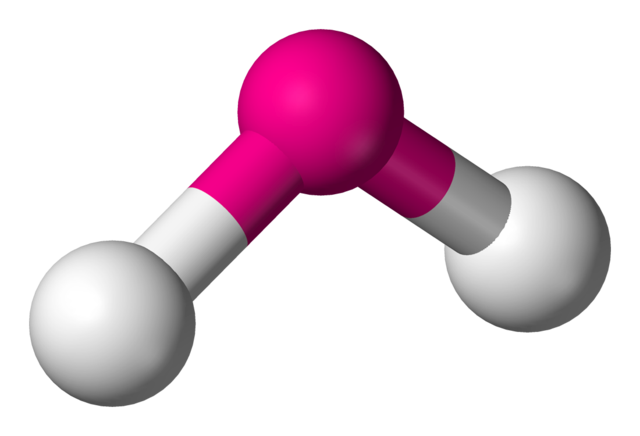
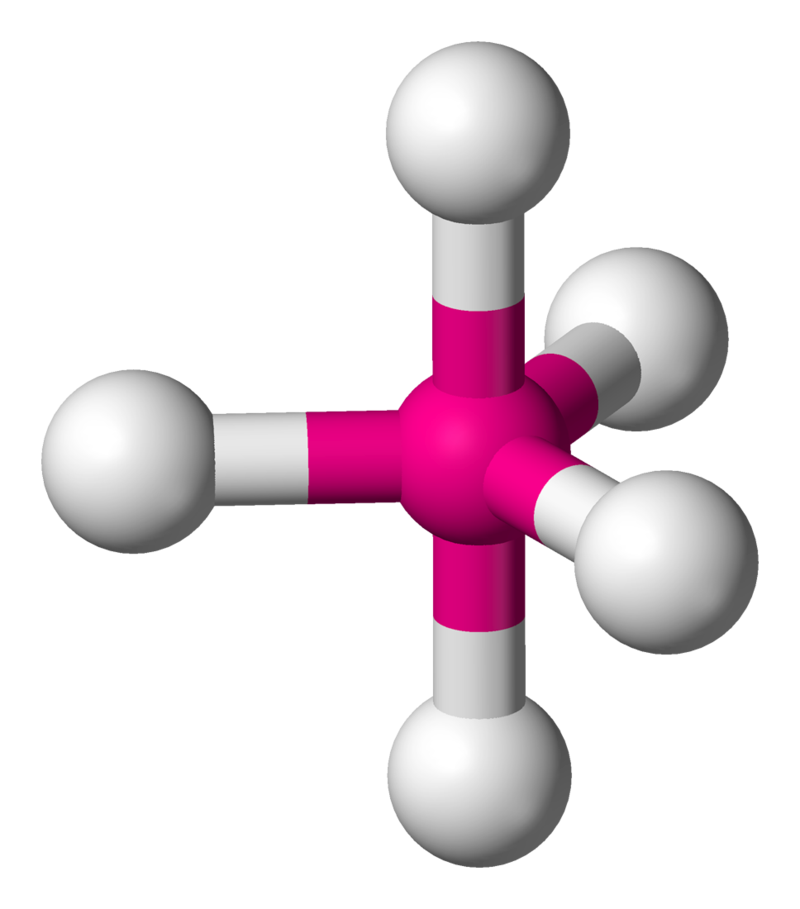
6. Trigonal Bipyramidal
- Example:
- Electron Pairs: 5 bonding pairs, 0 lone pairs.
- Bond Angles: 120° (equatorial) and 90° (axial).
- Explanation: Bonding pairs arrange in two planes to minimize repulsion.
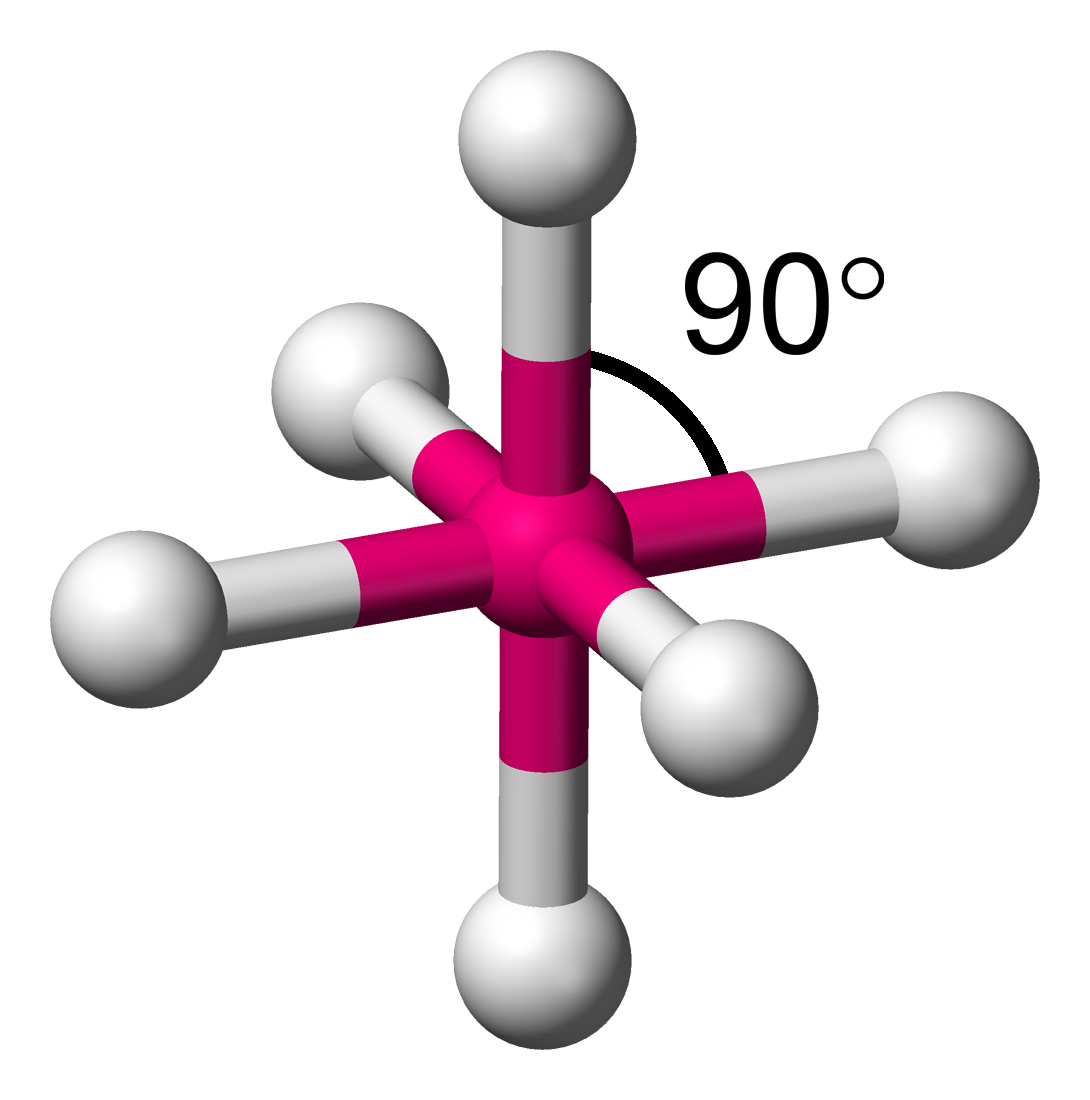
7. Octahedral
- Example:
- Electron Pairs: 6 bonding pairs, 0 lone pairs.
- Bond Angle: 90°.
- Explanation: The six bonding pairs arrange themselves symmetrically around the central atom.
8. Square Planar
- Example:
- Electron Pairs: 4 bonding pairs, 2 lone pairs.
- Bond Angle: 90°.
- Explanation: The lone pairs are placed opposite each other, leading to a flat, square shape.
Lone Pair Influence on Molecular Shapes
- Lone pairs take up more space than bonding pairs because they are only attracted to one nucleus. This leads to greater repulsion and smaller bond angles.
- Example: In water (H₂O), the bond angle is 104.5° due to the two lone pairs, which repel the bonding pairs more strongly.
Summary of Shapes Based on Electron Pairs
| Shape | Bonding Pairs | Lone Pairs | Bond Angle | Example |
|---|---|---|---|---|
| Linear | 2 | 0 | 180° | |
| Trigonal Planar | 3 | 0 | 120° | |
| Tetrahedral | 4 | 0 | 109.5° | |
| Trigonal Pyramidal | 3 | 1 | 107° | |
| Bent (V-Shaped) | 2 | 2 | 104.5° | |
| Trigonal Bipyramidal | 5 | 0 | 90° & 120° | |
| Octahedral | 6 | 0 | 90° | |
| Square Planar | 4 | 2 | 90° |
Exam Tip:
- Always count the total number of electron pairs around the central atom (both bonding and lone pairs).
- Use VSEPR theory to deduce the shape based on electron pair repulsion.
500K+ Students Use These Powerful Tools to Master Shapes of Simple Molecules & Ions For their A-Level Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
40 flashcards
Flashcards on Shapes of Simple Molecules & Ions
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards4 quizzes
Quizzes on Shapes of Simple Molecules & Ions
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes29 questions
Exam questions on Shapes of Simple Molecules & Ions
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Shapes of Simple Molecules & Ions
Create custom exams across topics for better practice!
Try Chemistry exam builder21 papers
Past Papers on Shapes of Simple Molecules & Ions
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Shapes of Simple Molecules & Ions you should explore
Discover More Revision Notes Related to Shapes of Simple Molecules & Ions to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Molecules: Shapes & Forces
Types of Forces Between Molecules
444+ studying
186KViews96%
114 rated
Molecules: Shapes & Forces
Effects of Forces Between Molecules
224+ studying
181KViews