Photo AI
Last Updated Sep 27, 2025
Hess' Law Simplified Revision Notes for A-Level AQA Chemistry
Revision notes with simplified explanations to understand Hess' Law quickly and effectively.
395+ students studying
1.6.5 Hess' Law
What is Hess' Law?
Hess' Law states that:
"The total enthalpy change of a reaction is the same, regardless of the route taken, provided that the initial and final conditions are the same."
This law is based on the principle of energy conservation, meaning that energy cannot be created or destroyed in a chemical reaction. Therefore, if a reaction can proceed through multiple routes, the total enthalpy change () will be identical, regardless of the path taken.
Why is Hess' Law Important?
Hess' Law is useful for calculating the enthalpy changes of reactions that cannot be measured directly. By constructing a cycle (commonly known as a Hess Cycle) that connects different routes, we can calculate the enthalpy change of the overall reaction using known enthalpy values for intermediate reactions.
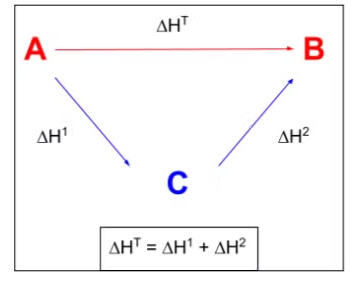
Using Hess' Law: The Triangular Cycle Method
One common method for applying Hess' Law is to use a triangular enthalpy cycle. This involves constructing a cycle of reactions, where:
- The arrows represent the enthalpy changes for different stages of the reaction.
- Direction of arrows: Indicates whether values should be added (for forward reactions) or subtracted (for reverse reactions). These arrows are treated like vectors in terms of their direction. The cycle connects the reactants to the products through a series of intermediate steps, such as formation or combustion processes.
500K+ Students Use These Powerful Tools to Master Hess' Law For their A-Level Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
70 flashcards
Flashcards on Hess' Law
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards7 quizzes
Quizzes on Hess' Law
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes29 questions
Exam questions on Hess' Law
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Hess' Law
Create custom exams across topics for better practice!
Try Chemistry exam builder21 papers
Past Papers on Hess' Law
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Hess' Law you should explore
Discover More Revision Notes Related to Hess' Law to Deepen Your Understanding and Improve Your Mastery
