Photo AI
Last Updated Sep 27, 2025
Calorimetry Simplified Revision Notes for A-Level AQA Chemistry
Revision notes with simplified explanations to understand Calorimetry quickly and effectively.
280+ students studying
1.6.4 Calorimetry
Calorimetry is the experimental technique used to measure the heat (or enthalpy) change in a chemical reaction. It helps determine how much energy is absorbed or released by a reaction. The heat change is represented by the symbol ( ), and it is essential for understanding the energetics of reactions such as combustion, neutralization, and solution processes.
The Heat Energy Equation
The heat change, (), in a reaction is given by the equation:
Where:
- (): mass of the substance (in grams) that experiences the temperature change.
- (): specific heat capacity (in ).
- (Delta T): temperature change (in Kelvin or degrees Celsius).
Applications
- You need to be able to use this equation to calculate the molar enthalpy change of a reaction and apply it to related calculations.
- You do not need to memorize the specific heat capacity () of substances; this value will be provided during experiments or in questions.
Example: Using the Calorimetry Equation to Calculate Enthalpy Change
Step 1: Calculate heat change () using the equation:
Step 2: Convert the heat change from joules () to kilojoules (), as standard enthalpies are typically expressed in
Step 3: Calculate the number of moles of the substance that caused this heat change using:
where is the molar mass of the substance.
Step 4: Calculate the enthalpy change per mole using the equation:
This gives the enthalpy change in
Types of Calorimetry Experiments
1. Reactions in Solution
For reactions in solution, such as neutralization:
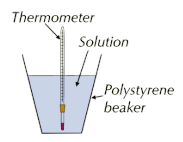
- The reactants are placed in a polystyrene cup (to reduce heat loss to the surroundings) inside a beaker for stability.
- A thermometer is used to measure the temperature change.
- Stirring ensures the solution is evenly heated.
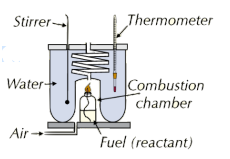
2. Combustion Reactions
For combustion reactions, the enthalpy change is determined by burning a flammable liquid in a calorimeter. The heat released by the burning fuel is absorbed by the water, and the temperature rise in the water is measured. However, not all the energy from the combustion is absorbed due to various limitations, such as:
- Heat loss to the surroundings.
- Incomplete combustion of the fuel.
- Evaporation of volatile fuels.
Errors and Accuracy in Calorimetry
Even with insulation, calorimetry experiments often have limitations:
- Heat loss to the surroundings.
- Incomplete combustion in reactions involving fuels.
- Evaporation of flammable liquids.
Improving Accuracy: Graphical Extrapolation
To calculate a more accurate temperature change:
- Measure the temperature at regular intervals before and after the reaction.
- Plot the temperature data on a graph.
- Draw lines of best fit for the temperatures before and after the reaction.
- Extrapolate both lines to the time when the reaction started. The difference between the two lines gives the accurate temperature change, accounting for heat lost to the surroundings.
Significant Figures and Units
When performing calorimetry calculations, it is important to:
- Use the correct units in
- Report answers to an appropriate number of significant figures, based on the precision of the measurements.
- Report results within the limits of the least accurate measurement provided.
500K+ Students Use These Powerful Tools to Master Calorimetry For their A-Level Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
70 flashcards
Flashcards on Calorimetry
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards7 quizzes
Quizzes on Calorimetry
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes29 questions
Exam questions on Calorimetry
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Calorimetry
Create custom exams across topics for better practice!
Try Chemistry exam builder21 papers
Past Papers on Calorimetry
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Calorimetry you should explore
Discover More Revision Notes Related to Calorimetry to Deepen Your Understanding and Improve Your Mastery
