Photo AI
Last Updated Sep 27, 2025
Effect of Concentration & Pressure Simplified Revision Notes for A-Level AQA Chemistry
Revision notes with simplified explanations to understand Effect of Concentration & Pressure quickly and effectively.
418+ students studying
1.7.5 Effect of Concentration & Pressure
The Effect of Concentration on Reaction Rate
In solutions, the concentration of reactants plays a crucial role in determining the rate of reaction. Concentration refers to the amount of reactant particles in a given volume. Increasing the concentration of a reactant generally increases the rate of reaction.
How Concentration Affects Collision Frequency
- Increased concentration means there are more particles in the same volume.
- With more particles present, the frequency of collisions between reactant particles increases.
- More collisions mean more opportunities for particles to collide with sufficient energy to overcome the activation energy, leading to an increase in the rate of successful reactions.
Maxwell-Boltzmann Distribution at Higher Concentration:
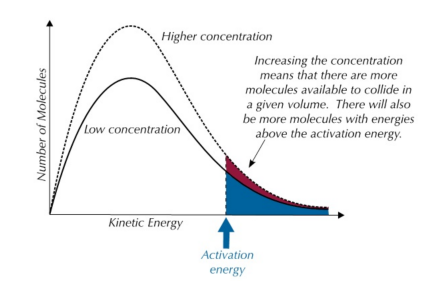
- The shape of the curve remains the same, meaning the most probable energy does not change.
- The peak of the curve stays at the same energy level but is higher because there are more molecules in total.
- The area under the curve increases, representing the larger number of reactant molecules.
Though increasing concentration raises the rate of reaction, its effect is less significant than the effect of increasing temperature. This is because only a slightly higher number of molecules gain enough energy to undergo successful collisions.
The Effect of Pressure on Reaction Rate (Gaseous Reactions)
For reactions involving gases, pressure plays a similar role to concentration. Increasing the pressure of a gas effectively increases the concentration of gas particles.
How Pressure Affects Collision Frequency
- Higher pressure compresses gas particles into a smaller volume, effectively increasing the number of particles per unit volume.
- This leads to more frequent collisions between gas particles.
- Like concentration, an increase in pressure increases the likelihood of successful collisions, thereby increasing the rate of reaction.
Pressure vs. Concentration
While both concentration (in solutions) and pressure (in gases) influence the rate of reaction by increasing the collision frequency, the underlying mechanism is the same: more particles in the same space leads to more collisions and, therefore, more chances for reactions to occur.
Summary
- Concentration: Increasing the concentration of reactants in a solution increases the number of collisions and, thus, the reaction rate. However, the effect is smaller compared to increasing temperature because only a slight increase in the number of molecules has sufficient energy for successful collisions.
- Pressure: In gaseous reactions, increasing pressure compresses the particles, increasing the frequency of collisions and the reaction rate in a similar way to concentration.
500K+ Students Use These Powerful Tools to Master Effect of Concentration & Pressure For their A-Level Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
60 flashcards
Flashcards on Effect of Concentration & Pressure
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards6 quizzes
Quizzes on Effect of Concentration & Pressure
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes29 questions
Exam questions on Effect of Concentration & Pressure
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Effect of Concentration & Pressure
Create custom exams across topics for better practice!
Try Chemistry exam builder21 papers
Past Papers on Effect of Concentration & Pressure
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Effect of Concentration & Pressure you should explore
Discover More Revision Notes Related to Effect of Concentration & Pressure to Deepen Your Understanding and Improve Your Mastery
