Photo AI
Last Updated Sep 27, 2025
Effect of Temperature on Reaction Rate Simplified Revision Notes for A-Level AQA Chemistry
Revision notes with simplified explanations to understand Effect of Temperature on Reaction Rate quickly and effectively.
493+ students studying
1.7.4 Effect of Temperature on Reaction Rate
Temperature plays a critical role in determining the rate of reaction. When the temperature of a reaction mixture is increased, the kinetic energy of the particles increases, leading to more frequent and energetic collisions between reactant molecules.
How Temperature Affects Molecular Energies
Increasing the temperature causes a shift in the Maxwell-Boltzmann distribution of molecular energies:
- At a higher temperature, the distribution curve shifts to the right and the peak becomes lower. This indicates that more molecules have higher kinetic energy.
- At a lower temperature, the curve shifts to the left and the peak becomes higher. This shows that most molecules have lower kinetic energy.
Important Features of the Maxwell-Boltzmann Distribution at Different Temperatures
- Higher temperatures:
- The distribution is broader and flatter, meaning more particles have higher energy.
- A larger number of molecules have energies greater than the activation energy, (), which is required for a reaction to occur.
- Lower temperatures:
- The distribution is narrower and the peak is higher, meaning most particles have lower energy.
- Fewer molecules have enough energy to surpass the activation energy.
Why Does Temperature Increase Reaction Rate?
A small increase in temperature can lead to a large increase in the rate of reaction. This occurs because:
- Increased kinetic energy: As temperature rises, particles move faster and collide more frequently.
- More successful collisions: A greater proportion of particles have energy equal to or greater than the activation energy, resulting in more successful collisions per unit of time.
Maxwell-Boltzmann Distribution Explanation
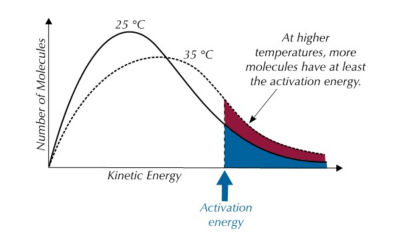
The area under the curve beyond the activation energy threshold () represents the number of molecules that can successfully react. At higher temperatures, this area increases significantly, meaning a larger proportion of particles have enough energy to undergo a reaction.
Thus, even a small increase in temperature leads to a large increase in reaction rate because the number of molecules with sufficient energy for a successful reaction increases exponentially.
Summary
- Increasing the temperature increases the kinetic energy of reactant molecules, shifting the Maxwell-Boltzmann distribution to the right.
- Higher temperatures result in more particles having enough energy to overcome the activation energy, leading to a faster reaction rate.
- The distribution at higher temperatures has a lower peak and more molecules with higher energy.
- Even a small temperature increase can cause a significant rise in reaction rate due to the exponential increase in the number of molecules with energy above the activation energy.
500K+ Students Use These Powerful Tools to Master Effect of Temperature on Reaction Rate For their A-Level Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
60 flashcards
Flashcards on Effect of Temperature on Reaction Rate
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards6 quizzes
Quizzes on Effect of Temperature on Reaction Rate
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes29 questions
Exam questions on Effect of Temperature on Reaction Rate
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Effect of Temperature on Reaction Rate
Create custom exams across topics for better practice!
Try Chemistry exam builder21 papers
Past Papers on Effect of Temperature on Reaction Rate
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Effect of Temperature on Reaction Rate you should explore
Discover More Revision Notes Related to Effect of Temperature on Reaction Rate to Deepen Your Understanding and Improve Your Mastery
