Photo AI
Last Updated Sep 27, 2025
Identification of Functional Groups by Test-Tube Reactions Simplified Revision Notes for A-Level AQA Chemistry
Revision notes with simplified explanations to understand Identification of Functional Groups by Test-Tube Reactions quickly and effectively.
495+ students studying
3.6.1 Identification of Functional Groups by Test-Tube Reactions
In organic chemistry, functional groups play a crucial role in determining the properties and reactions of compounds. This note will focus on how to identify key functional groups—alcohols, aldehydes, alkenes, and carboxylic acids—using simple test-tube reactions. These qualitative tests are essential for identifying unknown organic compounds.
Identifying Alcohols
Alcohols can be primary, secondary, or tertiary, and each behaves differently under oxidation conditions. The key reagent for testing alcohols is acidified potassium dichromate (VI).
Oxidation Test for Alcohols
- Reagent: Acidified potassium dichromate ( + )
- Procedure:
- Add 10 drops of the alcohol to 2 cm³ of acidified potassium dichromate solution.
- Warm the mixture gently in a water bath (avoid open flames).
- Results:
- Primary or Secondary Alcohol: Colour changes from orange () to green ().
- Tertiary Alcohol: No colour change (remains orange), as tertiary alcohols do not oxidise easily.
Reaction Diagrams:
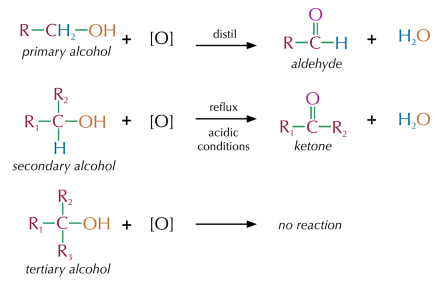
Distinguishing Primary from Secondary Alcohols
To differentiate between primary and secondary alcohols, you need to identify the oxidation product:
- Primary Alcohol:
- Under reflux, a primary alcohol oxidises to a carboxylic acid.
- Under distillation, a primary alcohol oxidises to an aldehyde.
- Secondary Alcohol:
- Under reflux, it oxidises to a ketone.
Identifying Aldehydes and Ketones
Aldehydes and ketones are carbonyl compounds. While both have the carbonyl () group, only aldehydes can be further oxidised.
Fehling's Solution Test
- Reagent: Fehling's solution (deep blue copper(II) complex)
- Procedure: 3. Add 2 cm³ of Fehling's solution to a test tube. 4. Add 5 drops of the unknown compound. 5. Warm the mixture in a water bath for 5 minutes.
- Results:
- Aldehyde: Blue solution turns brick-red due to the formation of a copper(I) oxide precipitate.
- Ketone: No colour change; remains blue.
Tollens' Reagent (Silver Mirror Test)
- Reagent: Tollens' reagent (ammoniacal silver nitrate)
- Procedure: 6. Add 2 cm³ of 0.10 mol dm⁻³ silver nitrate to a test tube. 7. Add a few drops of dilute sodium hydroxide—silver oxide forms as a brown precipitate. 8. Add dilute ammonia until the precipitate dissolves. 9. Warm the reagent in a water bath and add the unknown substance.
- Results:
- Aldehyde: A silver mirror forms on the inside of the test tube due to the reduction of silver ions to metallic silver.
- Ketone: No change; remains colourless.
Identifying Carboxylic Acids
Carboxylic acids can be distinguished from other organic acids due to their ability to react with carbonates, producing carbon dioxide gas.
Sodium Carbonate Test
- Reagent: Sodium carbonate ()
- Procedure: 10. Add 2 cm³ of the unknown substance to a test tube. 11. Add a small spatula of solid sodium carbonate or 2 cm³ of sodium carbonate solution. 12. If the solution fizzes, bubble the gas produced through limewater in a second test tube.
- Results:
- Carboxylic Acid: Fizzing ( production), and limewater turns cloudy.
- Non-Carboxylic Acid: No fizzing, limewater remains clear.
Limitation
This test will react positively with any acid, not just organic carboxylic acids. It is useful when you suspect the compound is an organic carboxylic acid.
Identifying Alkenes
Alkenes are unsaturated hydrocarbons containing a carbon-carbon double bond (). They can be easily identified using bromine water.
Bromine Water Test
- Reagent: Bromine water (orange solution of )
- Procedure: 13. Add 2 cm³ of the unknown solution to a test tube. 14. Add 2 cm³ of bromine water. 15. Shake the test tube.
- Results:
- Alkene: Orange solution decolourises, turning colourless due to the addition reaction with the double bond.
- Non-Alkene: No colour change, remains orange.
Summary of Tests for Functional Groups
| Functional Group | Test Reagent | Positive Result |
|---|---|---|
| Primary/Secondary Alcohol | Acidified | Orange to green (oxidation) |
| Tertiary Alcohol | Acidified | No colour change |
| Aldehyde | Fehling's/Tollens' | Brick-red precipitate / Silver mirror |
| Ketone | Fehling's/Tollens' | No change |
| Carboxylic Acid | Sodium Carbonate | Fizzing, limewater goes cloudy |
| Alkene | Bromine Water | Orange to colourless |
500K+ Students Use These Powerful Tools to Master Identification of Functional Groups by Test-Tube Reactions For their A-Level Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
30 flashcards
Flashcards on Identification of Functional Groups by Test-Tube Reactions
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards3 quizzes
Quizzes on Identification of Functional Groups by Test-Tube Reactions
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes29 questions
Exam questions on Identification of Functional Groups by Test-Tube Reactions
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Identification of Functional Groups by Test-Tube Reactions
Create custom exams across topics for better practice!
Try Chemistry exam builder21 papers
Past Papers on Identification of Functional Groups by Test-Tube Reactions
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Identification of Functional Groups by Test-Tube Reactions you should explore
Discover More Revision Notes Related to Identification of Functional Groups by Test-Tube Reactions to Deepen Your Understanding and Improve Your Mastery
