Photo AI
Last Updated Sep 27, 2025
Friedel–Crafts Acylation Simplified Revision Notes for A-Level AQA Chemistry
Revision notes with simplified explanations to understand Friedel–Crafts Acylation quickly and effectively.
310+ students studying
7.4.3 Friedel–Crafts Acylation
What is Acylation?
An acyl group consists of an alkyl group bonded to a carbon-oxygen double bond. Representing the alkyl group as "," the general formula for an acyl group is . Acylation refers to the process of introducing an acyl group into a molecule—in this case, into a benzene ring.
The most commonly used acyl group is , known as the ethanoyl group. In the following example, a group is added to the benzene ring, but any other alkyl group could be used in place of .
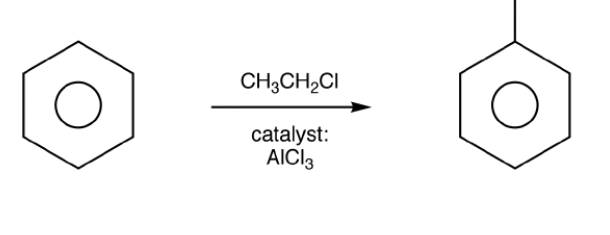
Friedel-Crafts Reactions
Friedel-Crafts reactions are a type of electrophilic substitution reaction. Due to the aromatic stability of arenes, these compounds are generally unreactive. To make arenes useful as starting materials for synthesizing other organic compounds, their structure must be altered to increase their reactivity.
Friedel-Crafts reactions achieve this by substituting a hydrogen atom on the benzene ring with either an alkyl group (Friedel-Crafts alkylation) or an acyl group (Friedel-Crafts acylation).
Like other electrophilic substitution reactions, Friedel-Crafts reactions proceed through three main steps:
- Generation of the electrophile
- Electrophilic attack on the benzene ring
- Restoration of the aromatic system in the benzene ring
- Regeneration of the Catalyst
The Friedel-Crafts acylation reaction involves attaching an acyl group to an aromatic ring. This is usually achieved using an acid chloride () along with a Lewis acid catalyst, such as **. During this reaction, the aromatic ring is converted into a ketone.
An acid anhydride can serve as an alternative to an acyl halide in Friedel-Crafts acylation reactions. In this reaction, the halogen in the acyl halide interacts with the Lewis acid, creating a highly electrophilic acylium ion (), which is stabilized by resonance.
Mechanism:
Friedel-Crafts acylations follow a four-step mechanism.
Step 1: Formation of the Electrophile
- Equation:
The aluminium chloride catalyst removes a chloride ion from methyl chloride
creating a highly reactive methyl carbocation ((\text{CH}_3^+)), which serves as an electrophile.
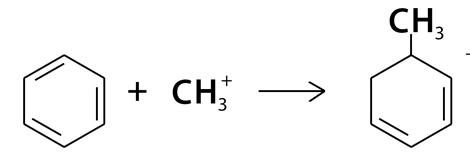
Step 2: Electrophilic Attack on Benzene
- The methyl carbocation attacks the electron-rich benzene ring. This breaks the aromaticity of benzene temporarily, resulting in the formation of a positively charged intermediate (a carbocation) where the methyl group is now attached to the benzene ring.
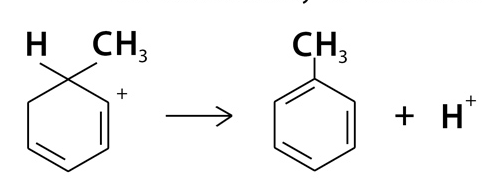
Step 3: Restoration of Aromaticity
- The positively charged intermediate releases a proton , which allows the benzene ring to regain its aromatic stability. This step completes the formation of methylbenzene. Here's the equation written out:
Step 4: Regeneration of the Catalyst
- The released proton reacts with the ion formed earlier, producing hydrochloric acid and regenerating the aluminium chloride catalyst which can then participate in further reactions.
Limitations
Although Friedel-Crafts acylation avoids some issues associated with Friedel-Crafts alkylation (such as carbocation rearrangement and polyalkylation), it still has a few drawbacks:
- Product Restriction: The reaction only produces ketones. This limitation arises because formyl chloride () decomposes into and under reaction conditions.
- Reactivity of the Aromatic Compound: Only aromatic compounds more reactive than mono-halobenzene can participate in this reaction. Less reactive compounds will not undergo acylation.
- Incompatibility with Aryl Amines: Aryl amines are unsuitable for this reaction because they form highly unreactive complexes with the Lewis acid catalyst.
- Side Reactions with Amines or Alcohols: When amines or alcohols are present, acylation may occur on the nitrogen or oxygen atoms instead of the aromatic ring.
Advantages:
Friedel-Crafts Acylation offers several advantages over Friedel-Crafts Alkylation. These include greater control over the reaction products and the stability of the acylium ion due to resonance, which eliminates the possibility of rearrangement. Additionally, the ketones produced through acylation can be reduced to alkyl groups using the Clemmensen reduction method.
Summary of Reactions of Arenes Table
| Reaction | Reaction Conditions | Products |
|---|---|---|
| Halogenation | / with /catalyst | Aryl halide |
| Nitration | Mixture of concentrated HNO₃ and concentrated at 25–60°C | Nitroarene |
| Friedel-Crafts alkylation | Halogenoalkane and anhydrous catalyst | Alkylbenzene |
| Friedel-Crafts acylation | Acyl chloride and anhydrous catalyst | Acylbenzene |
| Complete oxidation | Hot alkaline followed by dilute acid | Benzoic acid |
| Hydrogenation | Hydrogen with /catalyst and heat | Cyclohexane |
500K+ Students Use These Powerful Tools to Master Friedel–Crafts Acylation For their A-Level Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
30 flashcards
Flashcards on Friedel–Crafts Acylation
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards3 quizzes
Quizzes on Friedel–Crafts Acylation
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes29 questions
Exam questions on Friedel–Crafts Acylation
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Friedel–Crafts Acylation
Create custom exams across topics for better practice!
Try Chemistry exam builder21 papers
Past Papers on Friedel–Crafts Acylation
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Friedel–Crafts Acylation you should explore
Discover More Revision Notes Related to Friedel–Crafts Acylation to Deepen Your Understanding and Improve Your Mastery
