Photo AI
Last Updated Sep 27, 2025
Nitration of Benzene Simplified Revision Notes for A-Level AQA Chemistry
Revision notes with simplified explanations to understand Nitration of Benzene quickly and effectively.
484+ students studying
7.4.2 Nitration of Benzene
Reactions of Benzene
Despite containing carbon-carbon double bonds, benzene does not readily undergo addition reactions as might be expected. For instance, benzene does not decolorize bromine water and generally resists other addition reactions.
This resistance to addition reactions is due to the stability of the delocalized electron system in benzene. Any reaction that would disrupt this delocalized structure, such as addition reactions, is not energetically favorable. Instead, benzene tends to undergo substitution reactions, which preserve the stability of the delocalized electron ring.
Electrophilic Substitution Reactions of Benzene
Benzene's delocalized electrons create an electron-rich region that is attractive to electrophiles, making benzene prone to electrophilic substitution reactions. Two key electrophilic substitution reactions of benzene are nitration and halogenation.
Nitration of Benzene
Benzene reacts with a mixture of concentrated nitric acid ) and sulfuric acid ()** under reflux conditions at 50-55°C, producing nitrobenzene.
Reaction Steps:
- Formation of the Electrophile (NO₂⁺):
- Sulfuric acid, being a stronger acid than nitric acid, protonates nitric acid, allowing it to act as a base:
The unstable H₂NO₃⁺ ion then decomposes, forming water and the electrophile, NO₂⁺:
- Attack on the Benzene Ring:
- The NO₂⁺ ion acts as the electrophile and attacks the electron-rich benzene ring, temporarily breaking the delocalized structure.
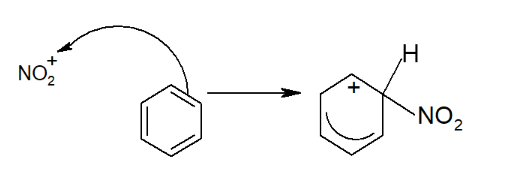
Step 3: The delocalized electron system is restored by drawing in electrons from the bond.
The H⁺ ion then combines with to regenerate .
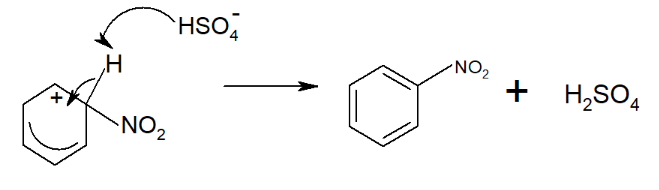
The overall reaction is à
It can also be written:
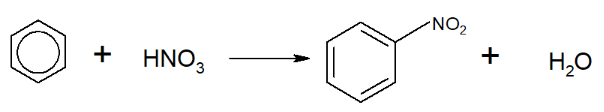
The sulphuric acid behaves as a catalyst. The product is known as nitrobenzene.
Acylation
Benzene reacts with acyl chlorides in the presence of anhydrous under reflux at 50°C to produce phenylketones. This process is known as the Friedel-Crafts acylation reaction.
Step 1: AlCl₃ acts as a Lewis acid, accepting a ion from the acyl chloride, which generates a carbocation:
The ion formed is the electrophile.
Step 2: This electrophile then attacks the electron-rich benzene ring, temporarily disrupting the delocalized electron system.
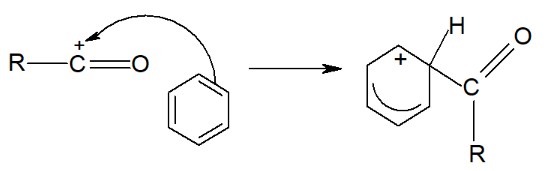
Step 3: The delocalized ring is restored by drawing electrons from the bond.
The H⁺ ion then reacts with , producing HCl gas and regenerating.
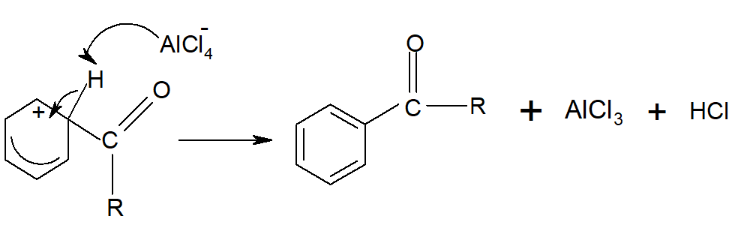
The overall reaction is therefore:
with AlCl₃ acting as a catalyst.
If ethanoyl chloride is used as the acyl chloride, the resulting product is phenylethanone.
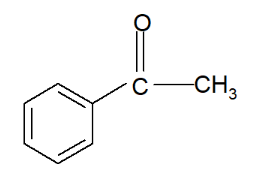
Further Substitution:
It is crucial to maintain the reaction temperature below 55°C to prevent further substitution from occurring.
500K+ Students Use These Powerful Tools to Master Nitration of Benzene For their A-Level Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
30 flashcards
Flashcards on Nitration of Benzene
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards3 quizzes
Quizzes on Nitration of Benzene
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes29 questions
Exam questions on Nitration of Benzene
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Nitration of Benzene
Create custom exams across topics for better practice!
Try Chemistry exam builder21 papers
Past Papers on Nitration of Benzene
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Nitration of Benzene you should explore
Discover More Revision Notes Related to Nitration of Benzene to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Aromatic Chemistry (A-level only)
Friedel–Crafts Acylation
427+ studying
200KViews