Photo AI
Last Updated Sep 27, 2025
Nucleophilic Substitution Simplified Revision Notes for A-Level AQA Chemistry
Revision notes with simplified explanations to understand Nucleophilic Substitution quickly and effectively.
389+ students studying
7.5.3 Nucleophilic Substitution
Nucleophilic Substitution
Nucleophilic substitution is a type of reaction in which a nucleophile replaces a leaving group in a compound. It plays a significant role in organic synthesis, particularly when working with halogenoalkanes, which are molecules where a halogen atom is bonded to an alkane.
Key Terms
- Nucleophile: A species with a negative or partial negative charge that donates a lone pair of electrons to form a covalent bond.
- Carbocation: An intermediate with a positively charged carbon atom, typically bonded to only three other atoms.
Nucleophilic Substitution in Halogenoalkanes
In nucleophilic substitution reactions, the nucleophile targets the positively charged carbon attached to the halogen in a halogenoalkane. This results in the replacement of the halogen by the nucleophile as the C–halogen bond breaks heterolytically, releasing the halide ion.
Three important nucleophilic substitution reactions involving halogenoalkanes include:
- Reaction with OH⁻ (Hydroxide):
- Nucleophile: from aqueous alkali solutions (e.g., or ).
- Product: An alcohol, as replaces the halogen.
- Mechanism: OH⁻ donates a pair of electrons to the partially positive carbon, forming a new C–O bond and releasing the halide ion.
- Condition: Aqueous and warm conditions.
- Reaction with (Cyanide):
- Nucleophile:from potassium or sodium cyanide in ethanol.
- Product: A nitrile, which lengthens the carbon chain by adding a carbon atom.
- Mechanism: attacks the carbon and replaces the halogen.
- Condition: Ethanol as the solvent and reflux conditions, as water would lead to the formation of an alcohol instead.
- Reaction with (Ammonia):
- Nucleophile: Ammonia ().
- Product: A primary amine, formed when replaces the halogen.
- Mechanism:
- NH₃ donates electrons to the carbon, forming a bond and resulting in a positively charged group.
- Another molecule or the halide ion then removes a proton from , forming the neutral amine.
- Conditions: Excess NH₃ in ethanol to prevent formation of further substituted amines and allow complete reaction to the primary amine.
Haloalkanes react with ammonia to produce an amine:
Halogenoalkane + ammonia → amine + ammonium halide
Conditions:
- Heat in a sealed flask with excess ammonia in ethanol.
- A sealed flask is used because the ammonia would escape as a gas if reflux was implemented.
Mechanism:
-
The ammonia has a lone pair of electrons and acts as a nucleophile attacking the of the polar bond. • The mechanism is nucleophilic substitution, as shown below:
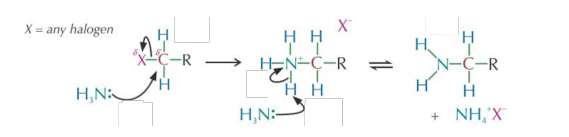
-
The amines produced from nucleophilic substitution reactions also have a lone pair of electrons on the atom.
-
These can act as a nucleophile and attack another haloalkane molecule, causing further substitution, continuing the reaction until a quaternary ammonium salt is made.
-
If excess ammonia is used then a primary amine is the major product.
-
If excess haloalkane is used then successive substitutions is more likely to occur - secondary, tertiary amines, and quaternary ammonia salts are the major products.
Cationic Surfactants
- Surfactants are compound which are partly soluble and partly insoluble in water.
- Some quaternary ammonium compounds can be used as cationic surfactants. These compounds are quaternary ammonium salts with at least one long hydrocarbon chain.
Cationic surfactants and cleaning up
- A long hydrocarbon chain is insoluble in water, but will bind to non-polar substances (like grease, soil, oil, etc).
- At the same time, the charged 'head' is soluble in water.
- This makes cationic surfactants useful as detergents; the non-polar end will bind to dirt and the polar head will dissolve in water.
- This allows us to wash away the dirt!
Amines as Nucleophiles in Nucleophilic Substitution
Amines are potent nucleophiles because the nitrogen atom has a lone pair of electrons, which can readily attack electrophilic carbon atoms. The nucleophilic substitution reactions of ammonia and amines with halogenoalkanes allow for the sequential formation of primary, secondary, and tertiary amines, as well as quaternary ammonium salts:
- Primary Amine Formation:
- Reaction between halogenoalkanes and excess ammonia yields a primary amine.
- Secondary and Tertiary Amine Formation:
- The primary amine formed in the first step can further react with more halogenoalkane to produce secondary and tertiary amines.
- Quaternary Ammonium Salt Formation:
- A tertiary amine can undergo one more nucleophilic substitution with a halogenoalkane to form a quaternary ammonium salt, where the nitrogen becomes positively charged and no longer has a lone pair.
Example: , where represents alkyl groups attached to nitrogen.
Use of Quaternary Ammonium Salts as Cationic Surfactants
Quaternary ammonium salts have practical applications as cationic surfactants due to their structure:
- Cationic Head: The positively charged nitrogen in the quaternary ammonium group attracts negatively charged surfaces (e.g., in detergents and fabric softeners).
- Hydrophobic Tail: The alkyl groups attached to the nitrogen are hydrophobic, helping these compounds act as surfactants that reduce surface tension and aid in cleaning.
Nucleophilic Addition-Elimination Reactions with Acyl Chlorides and Acid Anhydrides
Amines and ammonia can also undergo nucleophilic addition-elimination reactions with acyl chlorides and acid anhydrides. These reactions are common in the synthesis of amides.
- Reaction Mechanism with Acyl Chlorides:
- Nucleophile: Ammonia or primary amine.
- Mechanism:
- The nucleophile ( or amine) attacks the carbonyl carbon, forming a tetrahedral intermediate.
- This intermediate collapses, eliminating a halide ion () and forming an amide.
- Products:
- With Ammonia: A primary amide and as a by-product.
- With Primary Amine: A secondary amide and .
- Reaction Mechanism with Acid Anhydrides:
- Nucleophile: Ammonia or primary amine.
- Mechanism:
- The nucleophile attacks the carbonyl carbon in the anhydride, creating a tetrahedral intermediate.
- The intermediate collapses, splitting the anhydride and releasing a carboxylic acid by-product.
- Products:
- With Ammonia: Primary amide and a carboxylic acid.
- With Primary Amine: Secondary amide and a carboxylate ion.
Summary of Mechanisms
- Nucleophilic Substitution: Involves a nucleophile attacking an electrophilic carbon in a halogenoalkane, replacing the halogen.
- Nucleophilic Addition-Elimination: Occurs with acyl chlorides and acid anhydrides, where a nucleophile adds to the carbonyl carbon, followed by elimination to form an amide.
500K+ Students Use These Powerful Tools to Master Nucleophilic Substitution For their A-Level Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
50 flashcards
Flashcards on Nucleophilic Substitution
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards5 quizzes
Quizzes on Nucleophilic Substitution
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes29 questions
Exam questions on Nucleophilic Substitution
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Nucleophilic Substitution
Create custom exams across topics for better practice!
Try Chemistry exam builder21 papers
Past Papers on Nucleophilic Substitution
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Nucleophilic Substitution you should explore
Discover More Revision Notes Related to Nucleophilic Substitution to Deepen Your Understanding and Improve Your Mastery
