Photo AI
Last Updated Sep 27, 2025
Quaternary Ammonium Salts Simplified Revision Notes for A-Level AQA Chemistry
Revision notes with simplified explanations to understand Quaternary Ammonium Salts quickly and effectively.
310+ students studying
7.5.5 Quaternary Ammonium Salts
Quaternary Ammonium Salts
Quaternary ammonium salts are a type of ammonium compound where all four hydrogen atoms in the ammonium ion () have been replaced by alkyl groups. This results in a positively charged nitrogen atom with no remaining hydrogens directly attached. A common example is tetraethylammonium bromide, which is formed when triethylamin****e reacts with bromoethane.
Amines are produced from the reaction of a halogenoalkane with ammonia in a sealed tube. One mole of halogenoalkane reacts with two moles of ammonia producing a primary amine and an ammonium salt.
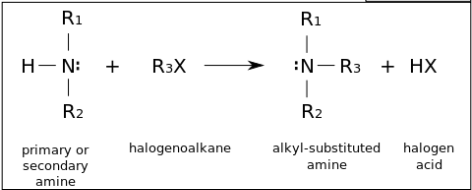
This substitution reaction can continue until all the hydrogen atoms have been replaced with organic groups. Following this, an additional substitution can occur, producing a quaternary ammonium salt.
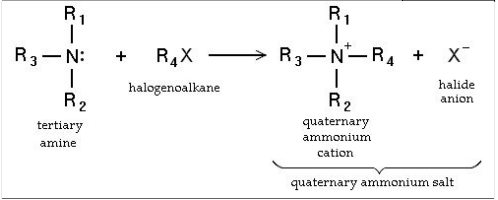
The multiple number of possible substitutions means that a mixture of products are produced. Therefore the reaction has low efficiency and reaction conditions have to be changed so that only a single substitution occurs. Ammonia can be added in excess in order to achieve only the primary amine, or the mixture of products can be separated using fractional distillation.
Formation of Quaternary Ammonium Salts
- The reaction occurs when an amine (such as triethylamine) reacts with a halogenoalkane (like bromoethane), replacing all hydrogens on nitrogen with alkyl groups.
- Final Stage: Once all four hydrogen atoms are replaced by alkyl groups, no further reactions can occur on the nitrogen, and the compound becomes a quaternary ammonium salt. For instance:
Here, tetraethylammonium bromide forms.
Structure and Properties
- Structure: Quaternary ammonium salts contain a nitrogen atom with a full positive charge (N⁺), surrounded by four alkyl groups, which may be identical or different.
- Analogy: They are structurally similar to ammonium chloride (), except that all hydrogens are substituted with alkyl chains, resulting in a stable positively charged species.
Uses in Industry
Quaternary ammonium salts are widely used in cleaning products, such as:
- Hair conditioners and fabric softeners, where they help reduce static and create a smooth texture on hair and fabric.
Cationic Surfactants
-
Hydrophilic and Hydrophobic Regions: The positively charged nitrogen end is hydrophilic, while the long alkyl (hydrocarbon) chains are hydrophobic.
- This allows the molecule to arrange itself at the water surface, with the hydrophilic end in the water and the hydrophobic tails pointing away from the water. This alignment reduces surface tension and enables the wetting process between immiscible liquids. Quaternary ammonium salts are effective cationic surfactants due to the following properties:
-
Cationic Charge: The nitrogen atom holds a positive charge, allowing these salts to act as cationic surfactants.
Mechanism of Action in Conditioners and Softeners
- When hair or fabric is wet, it can develop a negative charge that encourages static electricity buildup as it dries.
- Quaternary ammonium salts, with their positive charge, are attracted to these negatively charged surfaces.
- They form a coating over hair or fabric, resulting in a smooth finish and preventing static buildup, which improves the feel and appearance of both hair and fabric.
500K+ Students Use These Powerful Tools to Master Quaternary Ammonium Salts For their A-Level Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
50 flashcards
Flashcards on Quaternary Ammonium Salts
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards5 quizzes
Quizzes on Quaternary Ammonium Salts
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes29 questions
Exam questions on Quaternary Ammonium Salts
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Quaternary Ammonium Salts
Create custom exams across topics for better practice!
Try Chemistry exam builder21 papers
Past Papers on Quaternary Ammonium Salts
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Quaternary Ammonium Salts you should explore
Discover More Revision Notes Related to Quaternary Ammonium Salts to Deepen Your Understanding and Improve Your Mastery
