Photo AI
Last Updated Sep 27, 2025
Required Practical 8 Simplified Revision Notes for A-Level AQA Chemistry
Revision notes with simplified explanations to understand Required Practical 8 quickly and effectively.
305+ students studying
8.1.2 Required Practical 8
Aim:
To measure the electromotive force (EMF) of a zinc-copper electrochemical cell and compare the electrode potentials of different metals.
Equipment:
- Zinc metal strip
- Copper metal strip
- Emery paper or fine sandpaper
- Propanone (for cleaning)
- 1.0 mol dm⁻³ copper sulfate solution ()
- 1.0 mol dm⁻³ zinc sulfate solution ()
- 2.0 mol dm⁻³ sodium chloride solution ()
- U-tube or filter paper (salt bridge)
- Cotton wool
- Voltmeter
- Crocodile clips and wires
- Beakers (100 cm³ each)
Risks and Safety Precautions:
- Copper sulfate: Irritant. Wear gloves and avoid skin contact.
- Zinc sulfate: Irritant. Handle carefully, avoiding inhalation.
- Propanone: Flammable. Work in a well-ventilated area and keep away from open flames.
- General precautions: Wear eye protection and gloves throughout the experiment. In case of contact with skin or eyes, wash immediately with plenty of water.
Setting up an electrochemical cell: zinc and copper
Method:
- Prepare the metal electrodes:
- Clean the zinc and copper strips using emery paper to remove any oxide layer.
- Degrease both metals with cotton wool soaked in propanone to ensure good conductivity.
- Prepare the half-cells:
- Place the copper strip into a beaker containing 50 cm³ of 1.0 mol dm³ solution (forming the / half-cell).
- Place the zinc strip into another beaker with 50 cm³ of 1.0 mol dm³ solution (forming the / half-cell).
- Set up the salt bridge:
- Fill a U-tube with 2.0 mol dm³ solution, plugging both ends with cotton wool, ensuring the ends are submerged in each beaker. Alternatively, use filter paper soaked in solution as the salt bridge.
- Assemble the electrochemical cell:
- Connect the copper and zinc electrodes with crocodile clips and wires. Attach the leads to a voltmeter to measure the voltage of the electrochemical cell.
- Measure the EMF:
- Record the voltage displayed on the voltmeter. This is the EMF of the cell
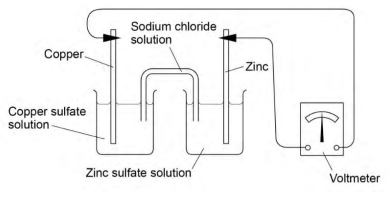
Salt Bridge Function:
- The salt bridge allows the movement of ions between the two half-cells, completing the circuit in an electrochemical cell.
- It must be inert, meaning it should not react with the electrolyte or ions present in the half-cells but must still conduct electricity.
- Commonly, platinum is used as the inert material, but in many experiments, a U-tube filled with solution is sufficient.
- Alternatively, a strip of filter paper soaked in sodium chloride () solution can be used as a salt bridge, which is simpler and effective in many setups.
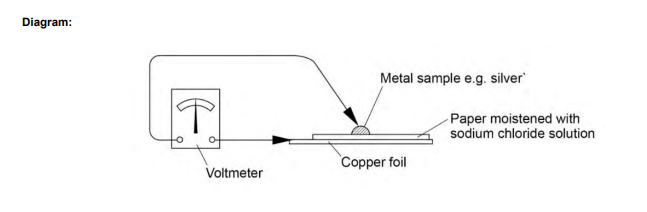
Comparing electrode potentials of different metals:
Method:
- Clean the copper electrode: Use emery paper or fine sandpaper to clean a piece of copper, removing any oxide layer.
- Connect the voltmeter: Attach one of the voltmeter leads to the copper using a crocodile clip, connecting it to the positive terminal.
- Prepare the filter paper: Cut a piece of filter paper roughly the same size as the copper piece, moisten it with sodium chloride () solution, and place it on top of the copper.
- Attach the second metal: Connect the second voltmeter lead to a different metal (e.g., zinc, silver) using a crocodile clip.
- Measure the potential difference: Press the second metal against the filter paper, and observe the voltage reading on the voltmeter, including the sign of the reading.
- Repeat: Use different metals in place of the second metal and compare their electrode potentials.
Conclusion:
- The EMF of an electrochemical cell depends on the difference in the electrode potentials of the two half-cells. A positive EMF value indicates the spontaneous direction of electron flow from the zinc (anode) to the copper (cathode).
- When different metals are used, the EMF will vary according to the standard electrode potentials of the metals. The larger the difference in electrode potentials, the higher the EMF.
Factors that affect the rate of mobility
- Affinity - pigments have different affinities to the chromatography paper; those with lower affinities will travel further up the paper.
- Solubility - pigments that are more soluble travel faster up the paper and will end up closer to the top at the solvent front. Pigments that travel further up the paper will have a higher Rf value
500K+ Students Use These Powerful Tools to Master Required Practical 8 For their A-Level Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
100 flashcards
Flashcards on Required Practical 8
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards10 quizzes
Quizzes on Required Practical 8
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes29 questions
Exam questions on Required Practical 8
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Required Practical 8
Create custom exams across topics for better practice!
Try Chemistry exam builder21 papers
Past Papers on Required Practical 8
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Required Practical 8 you should explore
Discover More Revision Notes Related to Required Practical 8 to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Physical Chemistry Practicals (A Level only)
Required Practical 7
246+ studying
200KViews96%
114 rated
Physical Chemistry Practicals (A Level only)
Required Practical 9
435+ studying
191KViews96%
114 rated
Physical Chemistry Practicals (A Level only)
Entropy of Vaporisation
231+ studying
189KViews96%
114 rated
Physical Chemistry Practicals (A Level only)
Iodine Clock Reaction
274+ studying
186KViews