Photo AI
Last Updated Sep 27, 2025
Iodine Clock Reaction Simplified Revision Notes for A-Level AQA Chemistry
Revision notes with simplified explanations to understand Iodine Clock Reaction quickly and effectively.
337+ students studying
8.1.5 Iodine Clock Reaction
Objective:
To investigate the effect of varying potassium iodide concentration on the initial rate of reaction between hydrogen peroxide and iodide ions using the "Iodine Clock" reaction. The rate equation for this reaction can be determined by measuring how long it takes for the reaction mixture to turn blue-black when starch is added as an indicator.
Chemical Reaction:
Hydrogen peroxide reacts with iodide ions in the presence of sulfuric acid to form iodine. Thiosulfate ions () are added to react immediately with the iodine as it forms, preventing a visible change until all thiosulfate is consumed. Once this occurs, the iodine reacts with starch, forming a blue-black complex:
Apparatus:
- 50 cm³ burette
- 250 cm³ beakers
- 50 cm³ measuring cylinder
- 25 cm³ measuring cylinder
- 100 cm³ beaker
- Plastic dropping pipette
- Stopwatch
- Stirring rod
Method:
- Prepare Potassium Iodide Solution:
- Rinse a 50 cm³ burette with potassium iodide solution, ensuring accuracy in all volumes.
- Fill the burette with potassium iodide solution.
- Transfer Hydrogen Peroxide:
- Using the burette, transfer 10.0 cm³ of hydrogen peroxide solution into a clean, dry 100 cm³ beaker.
- Add Sulfuric Acid:
- Use a 50 cm³ measuring cylinder to add 25 cm³ of 1.0 mol dm⁻³ sulfuric acid to a 250 cm³ beaker, ensuring the beaker is clean and dry.
- Add Water:
- Use a 25 cm³ measuring cylinder to add 20 cm³ of distilled (deionised) water into the 250 cm³ beaker.
- Add Starch Solution:
- Using a plastic dropping pipette, add 1.0 cm³ of starch solution to the same beaker.
- Starch acts as an indicator for the iodine produced in the reaction.
- Add Potassium Iodide Solution:
- Use the burette to add 5.0 cm³ of potassium iodide solution into the mixture in the 250 cm³ beaker.
- Add Sodium Thiosulfate:
- Add 5.0 cm³ of sodium thiosulfate solution from a burette into the same 250 cm³ beaker.
- Mix the Reaction Mixture:
- Stir the mixture thoroughly with a stirring rod.
- Pour the hydrogen peroxide solution from the 100 cm³ beaker into the 250 cm³ beaker, and immediately start the timer.
- Observe Colour Change:
- Stop the timer when the solution turns blue-black, indicating that all thiosulfate has been used up and free iodine is now present.
- Rinse Equipment:
-
Rinse the 250 cm³ beaker with distilled water and dry it with a paper towel. Repeat the Experiment:
-
Repeat the steps for a total of five experiments, varying the concentration of potassium iodide by adjusting the volume of water while keeping the total volume constant.
-
This allows you to determine the order of the reaction with respect to potassium iodide.
Plot a Graph:
- Plot a graph of the initial rate (1/time) against the concentration of potassium iodide to determine the reaction order with respect to iodide ions.
Improvements:
- Use a colourimeter to track the absorbance of iodine and minimise human error associated with timing the colour change manually.
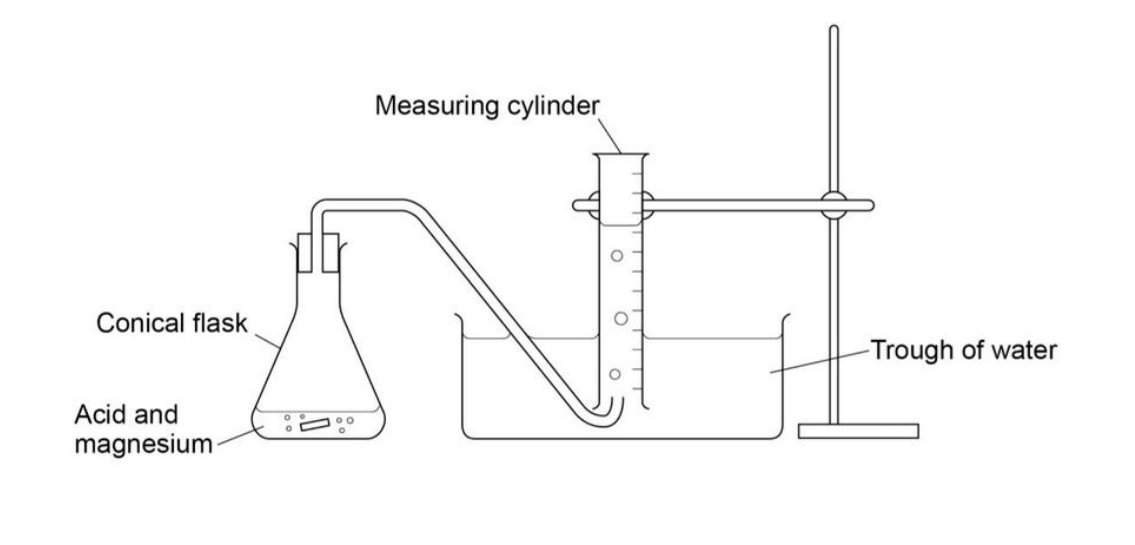
Continuous Monitoring Method:
- Add Hydrochloric Acid:
- Add 50 cm³ of 0.8 mol dm⁻³ hydrochloric acid into a conical flask.
- Set Up Gas Collection Equipment:
- Connect a gas syringe or set up alternative gas collection equipment to measure the volume of gas produced.
- Add Magnesium Ribbon:
- Add a 6 cm strip of magnesium ribbon into the flask and secure it with a bung. Immediately start the timer and swirl the flask gently.
- Record Hydrogen Gas Volume:
- Measure the volume of hydrogen gas produced every 15 seconds for 2.5 minutes.
- Alter Concentration of Hydrochloric Acid:
- Repeat steps (1) to (4) with varying concentrations of hydrochloric acid to observe how the rate of reaction changes.
Experiment Considerations:
- Ensure that the gas syringe has a capacity of at least 100 cm³ to prevent it from overfilling.
- The initial rate method is preferable for this reaction as it is easier to measure at the start when the concentration of reactants is known.
- In reactions involving multiple reactants, if the concentration of one reactant is in large excess, the reaction order with respect to that reactant will be zero because its concentration does not significantly change over time.
Analysis:
- Plot the volume of hydrogen produced over time for each concentration of hydrochloric acid.
- Draw a line of best fit for each concentration.
- Calculate the gradient of the line at time t = 0 to find the initial rate of reaction.
- Compare the rate values for different concentrations.
Other Ways to Follow the Reaction:
- Colourimeter: If any of the reactants or products are coloured (e.g., iodine), a colorimeter can measure absorbance over time. Absorbance is proportional to iodine concentration.
- Quenching: Aliquots of the reaction mixture can be withdrawn at intervals, quenched (by neutralising or cooling), and titrated to determine reactant concentration.
- Measuring Mass: Track mass lost during the reaction.
- Measuring pH: Monitor pH changes if the reaction produces acidic or basic products.
500K+ Students Use These Powerful Tools to Master Iodine Clock Reaction For their A-Level Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
100 flashcards
Flashcards on Iodine Clock Reaction
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards10 quizzes
Quizzes on Iodine Clock Reaction
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes29 questions
Exam questions on Iodine Clock Reaction
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Iodine Clock Reaction
Create custom exams across topics for better practice!
Try Chemistry exam builder21 papers
Past Papers on Iodine Clock Reaction
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Iodine Clock Reaction you should explore
Discover More Revision Notes Related to Iodine Clock Reaction to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Physical Chemistry Practicals (A Level only)
Required Practical 7
338+ studying
194KViews96%
114 rated
Physical Chemistry Practicals (A Level only)
Required Practical 8
387+ studying
190KViews96%
114 rated
Physical Chemistry Practicals (A Level only)
Required Practical 9
381+ studying
181KViews96%
114 rated
Physical Chemistry Practicals (A Level only)
Entropy of Vaporisation
464+ studying
192KViews