Photo AI
Last Updated Sep 27, 2025
Non-flow processes Simplified Revision Notes for A-Level AQA Physics
Revision notes with simplified explanations to understand Non-flow processes quickly and effectively.
435+ students studying
11.2.2 Non-flow processes
Non-flow processes are processes that occur in a closed system, where the gas does not move across the system's boundary. In these processes, no mass flows in or out. To apply the first law of thermodynamics to non-flow processes, we assume the gas behaves as an ideal gas.
Ideal Gas Assumption
An ideal gas is one that follows the gas laws perfectly, where:
- There are no interactions between gas molecules other than perfectly elastic collisions.
- No intermolecular forces act between molecules, which means no potential energy. Thus, the gas's internal energy depends solely on the kinetic energy of its particles. The ideal gas equation for a non-flow process is:
Where:
- = pressure,
- = volume,
- = number of moles of gas,
- = molar gas constant,
- = temperature in Kelvin. For a closed system, the amount of gas () remains constant, so .
This equation can also be expressed as:
which is a useful form for comparisons at two states.
Types of Non-Flow Processes
- Adiabatic Process
- An adiabatic process is where no heat enters or leaves the system, meaning .
- For an adiabatic process, the internal energy change ( $$\Delta U) is equal to the work done by/on the system.
- Ideal gas behaviour implies that internal energy depends only on temperature, so:
- When a gas expands (does work), temperature decreases.
- When a gas compresses (work is done on it), temperature increases. For adiabatic processes, the product of pressure and volume raised to the adiabatic constant is constant:
This can also be expressed as:
where depends on the type of gas.
- Isothermal Process
- An isothermal process is where temperature remains constant, so .
- Here, all energy transfer is in the form of work done by/on the gas i.e.,.
- The product of pressure and volume remains constant, following Boyle's Law:
This is expressed as:
- Constant Pressure Process
- In a constant pressure process, pressure remains constant as volume changes.
- The work done () can be calculated using:
where is the change in volume.
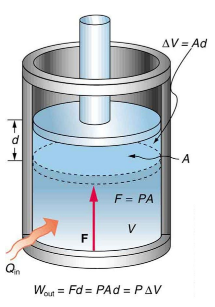
Derivation:
Work done can be derived using the formula for force and area, assuming a piston system:
- Since (pressure times area),
- And (distance moved times area is volume change),
- This gives .
- Constant Volume Process
- In a constant volume process, the volume remains constant. Hence, no work is done by or on the system .
- According to the first law of thermodynamics:
This implies that all energy transfer results in a change in internal energy, affecting the temperature.
- If energy is added, temperature increases.
- If energy is removed, temperature decreases.
Applications of Non-Flow Processes
Understanding these processes is essential in thermodynamics, especially for systems where gas is heated or cooled in closed conditions (e.g., piston engines, closed cylinders).
500K+ Students Use These Powerful Tools to Master Non-flow processes For their A-Level Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
60 flashcards
Flashcards on Non-flow processes
Revise key concepts with interactive flashcards.
Try Physics Flashcards6 quizzes
Quizzes on Non-flow processes
Test your knowledge with fun and engaging quizzes.
Try Physics Quizzes29 questions
Exam questions on Non-flow processes
Boost your confidence with real exam questions.
Try Physics Questions27 exams created
Exam Builder on Non-flow processes
Create custom exams across topics for better practice!
Try Physics exam builder56 papers
Past Papers on Non-flow processes
Practice past papers to reinforce exam experience.
Try Physics Past PapersOther Revision Notes related to Non-flow processes you should explore
Discover More Revision Notes Related to Non-flow processes to Deepen Your Understanding and Improve Your Mastery
