Photo AI
Last Updated Sep 27, 2025
Second Law and Engines Simplified Revision Notes for A-Level AQA Physics
Revision notes with simplified explanations to understand Second Law and Engines quickly and effectively.
307+ students studying
11.2.5 Second Law and Engines
Second Law of Thermodynamics
The second law of thermodynamics states that a heat engine must operate between a source and a sink to function. This implies that:
- The engine receives heat from a high-temperature source.
- The engine does work (W) as part of its operation.
- A portion of the heat energy is inevitably lost to a sink at a lower temperature. In other words, some energy must be transferred to the sink and cannot be used to perform work, which makes it impossible for the engine to achieve 100% efficiency. For example, if the engine and source reached the same temperature, heat transfer would stop, and the engine would cease to do work.
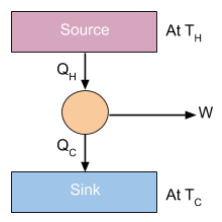
Efficiency of Heat Engines
The efficiency of a heat engine can be calculated as:
where:
- : Heat energy absorbed from the source.
- : Heat energy lost to the sink.
- : Work output. The maximum theoretical efficiency (for an ideal engine using an ideal gas) is determined by the temperatures of the source and sink (:
where:
-
and must be in Kelvin. To maximise engine efficiency:
-
Source temperature ( should be as high as possible.
-
Sink temperature should be as low as possible, ideally reaching absolute zero (0 K) for perfect efficiency, although this is unachievable in practical systems.
Real Engine Efficiency Limitations
Real engines cannot achieve the theoretical maximum efficiency for several reasons:
- Frictional forces within the engine consume some energy.
- Incomplete combustion of fuel leads to less effective heating.
- Internal components (e.g., pumps, motors) require energy to operate.
Enhancing Efficiency in Practical Systems
Despite the limitations, there are practical approaches to optimise energy use:
- Combined Heat and Power (CHP) Schemes:
- These systems capture and use waste heat (e.g., from power plants) to heat nearby buildings. This approach is efficient when facilities are close to populated areas, although distance limits practical application in the UK.
- Regenerative Braking:
- In vehicles, braking energy (usually lost as heat) is stored, for example, in a flywheel. This stored energy can later be used to accelerate the vehicle, thereby conserving energy that would otherwise dissipate.
500K+ Students Use These Powerful Tools to Master Second Law and Engines For their A-Level Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
60 flashcards
Flashcards on Second Law and Engines
Revise key concepts with interactive flashcards.
Try Physics Flashcards6 quizzes
Quizzes on Second Law and Engines
Test your knowledge with fun and engaging quizzes.
Try Physics Quizzes29 questions
Exam questions on Second Law and Engines
Boost your confidence with real exam questions.
Try Physics Questions27 exams created
Exam Builder on Second Law and Engines
Create custom exams across topics for better practice!
Try Physics exam builder56 papers
Past Papers on Second Law and Engines
Practice past papers to reinforce exam experience.
Try Physics Past PapersOther Revision Notes related to Second Law and Engines you should explore
Discover More Revision Notes Related to Second Law and Engines to Deepen Your Understanding and Improve Your Mastery
