Photo AI
Last Updated Sep 27, 2025
Nuclear Radius Simplified Revision Notes for A-Level AQA Physics
Revision notes with simplified explanations to understand Nuclear Radius quickly and effectively.
346+ students studying
8.1.5 Nuclear Radius
Understanding Nuclear Radius
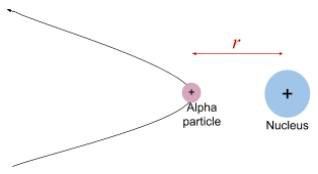
The nuclear radius of an atom can be estimated by calculating the distance of closest approach of a charged particle, such as an alpha particle, fired at a gold nucleus.
Distance of Closest Approach:
- As the alpha particle moves towards the positively charged nucleus, it experiences a repulsive electrostatic force.
- The particle's kinetic energy is converted into electric potential energy as it slows down.
- The point at which the alpha particle stops and has zero kinetic energy marks the distance of closest approach, symbolised as .
- The electric potential at this point is given by:
Where:
- is the permittivity of free space,
- is the charge of the nucleus,
- is the distance of closest approach.
Electric Potential Energy Calculation:
- Electric potential energy at this distance can be calculated as:
- This energy represents the work done to bring the charges to this distance apart.
Electron Diffraction as an Alternative Method:
- Electron diffraction offers a more accurate measure of nuclear radius as it avoids interaction with the strong nuclear force.
- Electrons, being leptons, do not experience this force, unlike alpha particles, providing a clearer measure.
- High-speed electrons with a De Broglie wavelength around 10^-15 m pass through a thin material, creating a diffraction pattern.
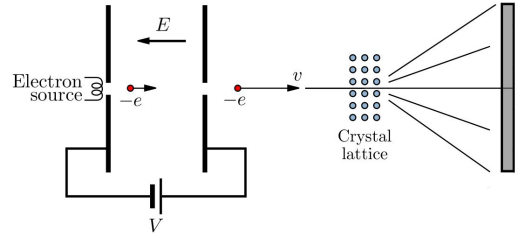
Diffraction Pattern Analysis:
- The pattern observed is a series of concentric circles.
- The intensity of these circles diminishes as distance from the centre increases.
- By plotting a graph of intensity against diffraction angle, we can measure the diffraction angle of the first minimum to estimate nuclear radius using:

Where:
- is the diffraction angle,
- is the electron's De Broglie wavelength,
- is the nuclear radius.
Graphical Method for Nuclear Radius:
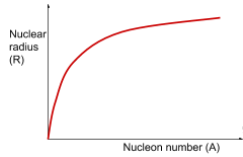
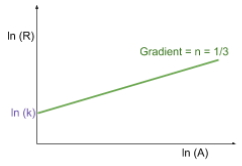
- A logarithmic plot of nuclear radius against nucleon number (mass number) provides further insight:
- Taking logs, we get:
- Plotting against , the gradient of this line (approximately 1/3) and the intercept help determine the relationship.
Nuclear Density:
- Using the above relationship, it can be shown that nuclear density is constant across all nuclei.
- Calculation shows nuclear density around 1.45 × 10^17 kg/m³, which indicates that most of an atom's mass is concentrated in the nucleus, with the rest being mostly empty space.
500K+ Students Use These Powerful Tools to Master Nuclear Radius For their A-Level Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
80 flashcards
Flashcards on Nuclear Radius
Revise key concepts with interactive flashcards.
Try Physics Flashcards8 quizzes
Quizzes on Nuclear Radius
Test your knowledge with fun and engaging quizzes.
Try Physics Quizzes29 questions
Exam questions on Nuclear Radius
Boost your confidence with real exam questions.
Try Physics Questions27 exams created
Exam Builder on Nuclear Radius
Create custom exams across topics for better practice!
Try Physics exam builder56 papers
Past Papers on Nuclear Radius
Practice past papers to reinforce exam experience.
Try Physics Past PapersOther Revision Notes related to Nuclear Radius you should explore
Discover More Revision Notes Related to Nuclear Radius to Deepen Your Understanding and Improve Your Mastery
