Photo AI
Last Updated Sep 27, 2025
Rutherford scattering Simplified Revision Notes for A-Level AQA Physics
Revision notes with simplified explanations to understand Rutherford scattering quickly and effectively.
325+ students studying
8.1.1 Rutherford scattering
Key Concept
Rutherford scattering provided critical evidence for the existence of a nucleus within the atom. This was a breakthrough as it challenged the previously accepted plum pudding model proposed by J.J. Thomson.
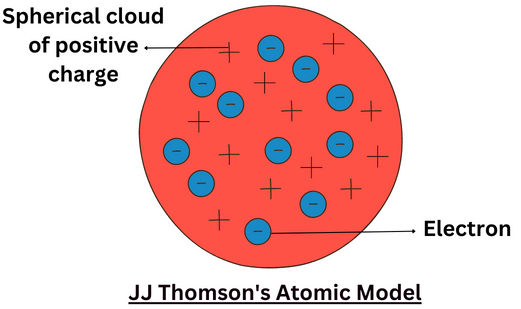
Thomson's Plum Pudding Model
Before Rutherford's experiment, it was widely accepted that an atom consisted of a sphere of positive charge with negative charges (electrons) distributed evenly within it—similar to "plums in a pudding."
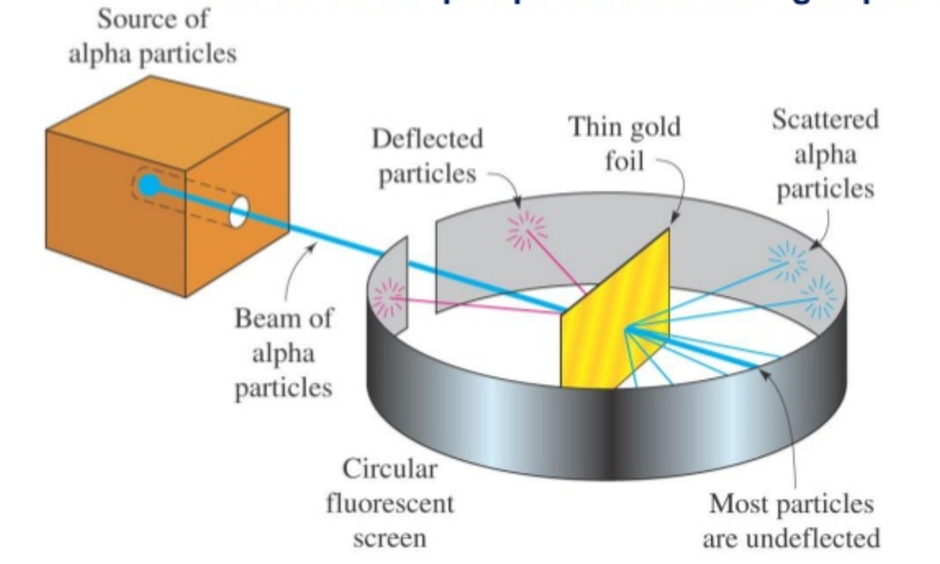
Rutherford's Experiment Setup
- Alpha Particles: A source emitting alpha particles was aimed at a very thin sheet of gold foil placed inside a chamber.
- Detection: The chamber was coated with a fluorescent material that would emit light upon being struck by alpha particles, making it possible to observe their paths.
- Observation: A microscope was used to examine the scattering angles of the alpha particles after they struck the foil.
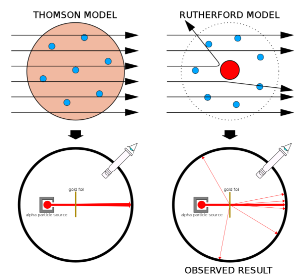
Expected vs. Observed Results
If the plum pudding model were accurate, most alpha particles would pass through with minimal deflection, as the charge in the atom would be spread out evenly.
However, Rutherford observed:
- Most alpha particles passed straight through with no deflection, indicating that atoms are mostly empty space.
- A small fraction of particles were deflected at large angles, suggesting a concentrated positive charge repelling them.
- A very few particles were deflected back by angles greater than 90°, implying that the positive charge was in a small, dense central region.
Conclusion: The Nuclear Model
Rutherford's findings led to a new model, known as the nuclear model:
- The atom has a small, dense nucleus that is positively charged.
- Electrons exist outside the nucleus, meaning that most of the atom is empty space.
500K+ Students Use These Powerful Tools to Master Rutherford scattering For their A-Level Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
80 flashcards
Flashcards on Rutherford scattering
Revise key concepts with interactive flashcards.
Try Physics Flashcards8 quizzes
Quizzes on Rutherford scattering
Test your knowledge with fun and engaging quizzes.
Try Physics Quizzes29 questions
Exam questions on Rutherford scattering
Boost your confidence with real exam questions.
Try Physics Questions27 exams created
Exam Builder on Rutherford scattering
Create custom exams across topics for better practice!
Try Physics exam builder56 papers
Past Papers on Rutherford scattering
Practice past papers to reinforce exam experience.
Try Physics Past PapersOther Revision Notes related to Rutherford scattering you should explore
Discover More Revision Notes Related to Rutherford scattering to Deepen Your Understanding and Improve Your Mastery
