Photo AI
Last Updated Sep 27, 2025
Boyle's and Charles's Laws Simplified Revision Notes for A-Level AQA Physics
Revision notes with simplified explanations to understand Boyle's and Charles's Laws quickly and effectively.
253+ students studying
Boyle's and Charles's Laws
Part 1: Boyle's Law
Boyle's Law states that the pressure of a gas is inversely proportional to its volume when temperature is constant. Mathematically:
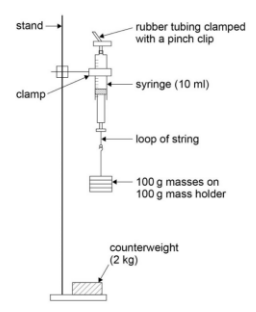
Equipment
- Stand and clamp: To hold the apparatus securely.
- Syringe: To contain a fixed amount of air.
- Rubber tubing and pinch clip: To seal the syringe and prevent air escape.
- String: To suspend weights from the syringe.
- 100g masses with holder: For applying force to the syringe plunger.
Method
- Measure Cross-Sectional Area:
- With the plunger removed, measure the internal diameter of the syringe using a vernier calliper. Calculate the cross-sectional area using:
- Initial Setup:
- Reinsert the plunger and draw in about 4.0 ml of air. Record this initial volume .
- Attach rubber tubing to the syringe nozzle, and secure it with a pinch clip to prevent air escape.
- Apply Masses and Record Volumes:
- Set up the apparatus with a 100g mass on the syringe holder.
- Gently move the plunger to ensure it is not sticking, then release it to record the volume .
- Gradually add 100g masses, recording the volume each time until the total mass reaches 1000g.
- Repeat:
- Perform the experiment twice more to find the average volume for each mass applied.
Graphs and Calculations
- Calculate Pressure:
- Calculate the force exerted by each mass using .
- Compute the pressure exerted by this force on the gas:
- Subtract standard atmospheric pressure (101 kPa) to obtain the pressure due to the applied masses alone.
- Graph of vs. :
- Plot against and draw a line of best fit. The linear relationship confirms Boyle's Law.
Safety
- Stable Stand: Use a counterweight to prevent the stand from tipping over due to the hanging weights.
Improvements and Notes
- Avoid Distortion: Ensure the clamp does not deform the syringe, as this affects volume measurements.
- Lubricate the Plunger: To avoid sticking, which could lead to inaccurate volume readings.
Part 2: Charles's Law
Charles's Law states that the volume of a gas is directly proportional to its absolute temperature when pressure is constant. Mathematically:
Equipment
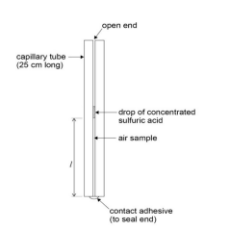
- Capillary tube (sealed at one end): Contains the air sample.
- Sulfuric acid: Used to trap the air sample in the capillary tube.
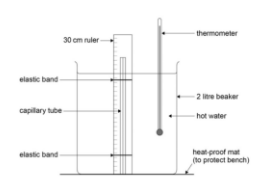
- 2-litre beaker: For heating the water.
- Thermometer: To measure the temperature of water.
- Kettle: To heat the water.
- 30 cm ruler: For measuring the length of the air column in the capillary tube.
Method
- Setup:
- Place the capillary tube in the beaker with the open end above the water.
- Pour hot water from the kettle into the beaker until the air sample in the tube is fully covered.
- Heat and Measure Volume:
- Stir the water and record its temperature and the length of the trapped air column.
- Allow the water to cool by 5°C, and repeat measurements of and until reaching room temperature.
Graphs and Calculations
- Graph of vs. :
- Plot length of the air column against temperature and draw a line of best fit.
- The gradient and intercept allow calculation of absolute zero. Using the equation:
Absolute zero can be estimated by setting giving:
Safety
- Chemical Handling: Wear safety goggles to avoid eye contact with sulfuric acid.
- Hot Water: Take care with boiling water to avoid burns.
Improvements and Notes
- Chemical Purity: Ensure the capillary tube is free from any contaminants that may react with sulfuric acid.
- Accurate Measurements: The sulfuric acid thread must be fully intact to accurately trap the air column.
Key Concepts
- Boyle's Law: Demonstrates the inverse relationship between pressure and volume at constant temperature.
- Charles's Law: Shows the direct relationship between volume and temperature at constant pressure.
- Absolute Zero: By extrapolating the graph of Charles's Law, we can estimate the temperature at which the volume of a gas theoretically becomes zero.
500K+ Students Use These Powerful Tools to Master Boyle's and Charles's Laws For their A-Level Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
130 flashcards
Flashcards on Boyle's and Charles's Laws
Revise key concepts with interactive flashcards.
Try Physics Flashcards13 quizzes
Quizzes on Boyle's and Charles's Laws
Test your knowledge with fun and engaging quizzes.
Try Physics Quizzes29 questions
Exam questions on Boyle's and Charles's Laws
Boost your confidence with real exam questions.
Try Physics Questions27 exams created
Exam Builder on Boyle's and Charles's Laws
Create custom exams across topics for better practice!
Try Physics exam builder56 papers
Past Papers on Boyle's and Charles's Laws
Practice past papers to reinforce exam experience.
Try Physics Past PapersOther Revision Notes related to Boyle's and Charles's Laws you should explore
Discover More Revision Notes Related to Boyle's and Charles's Laws to Deepen Your Understanding and Improve Your Mastery
