Photo AI
Last Updated Sep 26, 2025
Metals as Conductors Simplified Revision Notes for GCSE AQA Chemistry Combined Science
Revision notes with simplified explanations to understand Metals as Conductors quickly and effectively.
287+ students studying
2.2.9 Metals as Conductors
Metals are excellent conductors of both heat and electricity, and this is due to their unique structure and the behaviour of their electrons.
Delocalised Electrons:
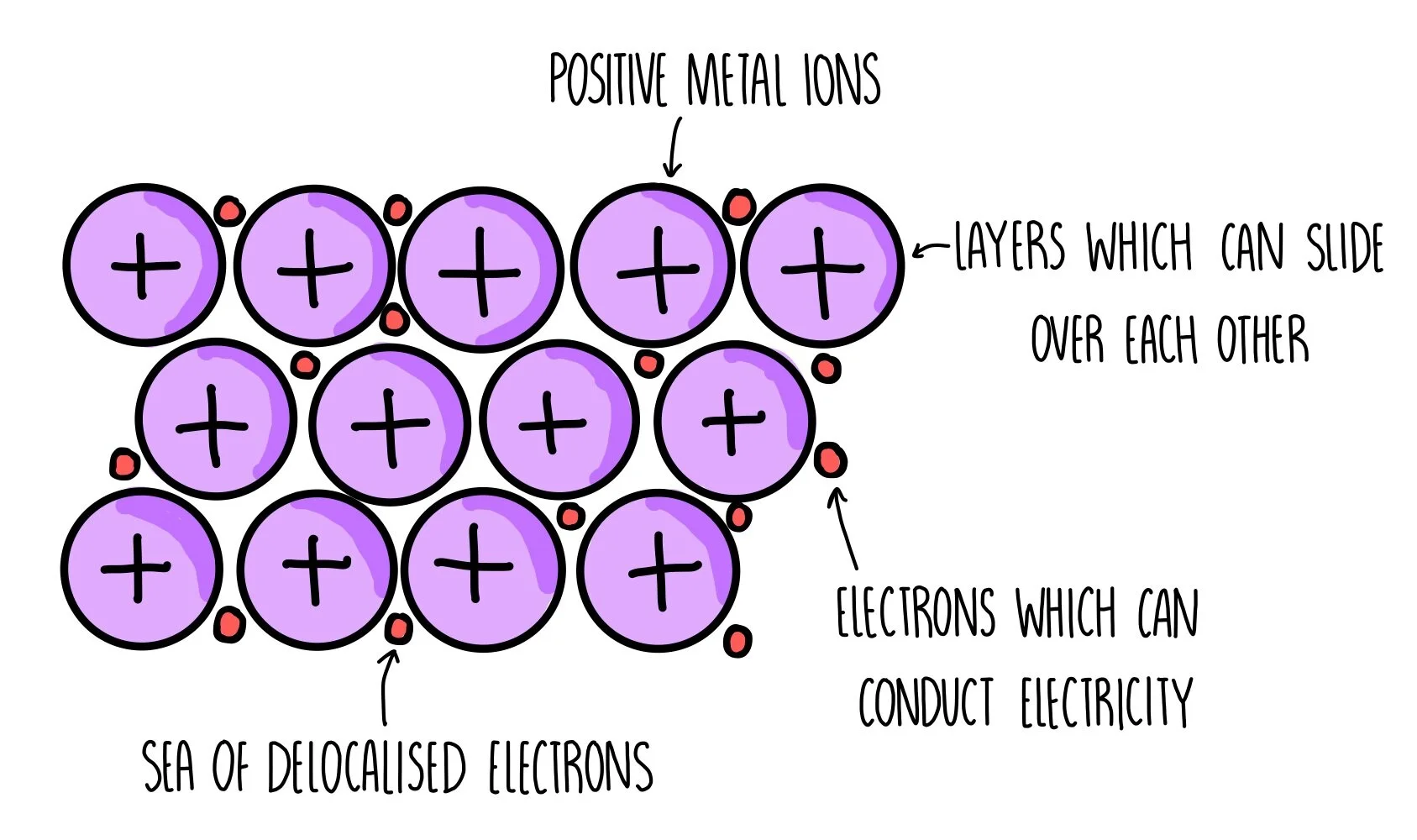
- In metals, some of the electrons are not tied to a specific atom. Instead, they are delocalised, meaning they can move freely throughout the metal's structure. These delocalised electrons are key to why metals can conduct electricity and heat so well.
- Electrical Conductivity: When a metal is connected to a power source, the delocalised electrons move in a specific direction, carrying an electric charge through the metal. This flow of electrons is what we know as an electric current.
- Thermal Conductivity: Metals are also good at conducting heat. When one part of a metal is heated, the delocalised electrons gain energy and move faster. They quickly transfer this energy throughout the metal, causing the entire piece to heat up evenly.
Metallic Bonding:
The reason metals have these free-moving, delocalised electrons is due to their metallic bonding.
What is Metallic Bonding?
In metals, atoms are arranged in a regular pattern, and they release some of their electrons to form a "sea of electrons" that surrounds the positively charged metal ions. The attraction between these positive ions and the sea of delocalised electrons holds the metal together.
Why It Matters: This bonding gives metals their key properties:
- Malleability: The layers of atoms in a metal can slide over each other without breaking the metallic bond, which is why metals can be hammered into shapes.
- Ductility: Similarly, metals can be drawn into wires because the metallic bonds remain intact as the atoms slide past each other.
Examples of Metal Conductors:
- Copper: Copper is commonly used in electrical wiring because it has very high electrical conductivity and is relatively inexpensive.
- Aluminium: Used in power lines and electrical cables because it's lightweight and still a good conductor of electricity.
- Gold and Silver: Although expensive, they are used in high-end electronics because they are even better conductors than copper and do not corrode.
Importance of Metals as Conductors:
- Electronics and Wiring: Metals are essential in making wires, circuits, and electronic components because they efficiently carry electricity to where it's needed.
- Cooking Utensils: Metals like aluminium and stainless steel are used in pots and pans because they quickly and evenly distribute heat, making them ideal for cooking.
Summary:
Metals are good conductors of heat and electricity because of the presence of delocalised electrons that can move freely throughout the metal. These electrons carry energy and charge, making metals crucial in a wide range of applications, from electrical wiring to cookware.
500K+ Students Use These Powerful Tools to Master Metals as Conductors For their GCSE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
100 flashcards
Flashcards on Metals as Conductors
Revise key concepts with interactive flashcards.
Try Chemistry Combined Science Flashcards10 quizzes
Quizzes on Metals as Conductors
Test your knowledge with fun and engaging quizzes.
Try Chemistry Combined Science Quizzes29 questions
Exam questions on Metals as Conductors
Boost your confidence with real exam questions.
Try Chemistry Combined Science Questions27 exams created
Exam Builder on Metals as Conductors
Create custom exams across topics for better practice!
Try Chemistry Combined Science exam builder25 papers
Past Papers on Metals as Conductors
Practice past papers to reinforce exam experience.
Try Chemistry Combined Science Past PapersOther Revision Notes related to Metals as Conductors you should explore
Discover More Revision Notes Related to Metals as Conductors to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Bonding & Substance Properties
Particle Theory & its Limitations
346+ studying
198KViews96%
114 rated
Bonding & Substance Properties
Properties of Ionic Compounds
365+ studying
182KViews