Photo AI
Last Updated Sep 26, 2025
Mixtures Simplified Revision Notes for GCSE AQA Chemistry
Revision notes with simplified explanations to understand Mixtures quickly and effectively.
330+ students studying
1.1.5 Mixtures
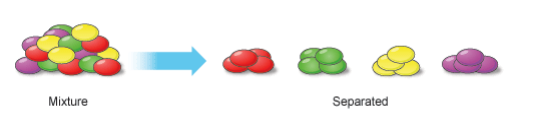
A mixture is made from different substances that are not chemically joined.
Example:
- Powdered iron and powdered sulfur mixed together form a mixture of iron and sulfur.
- They can be separated from each other without a chemical reaction, just like different colored sweets can be picked out from a mixed packet and sorted into separate piles.
Types of Substances:
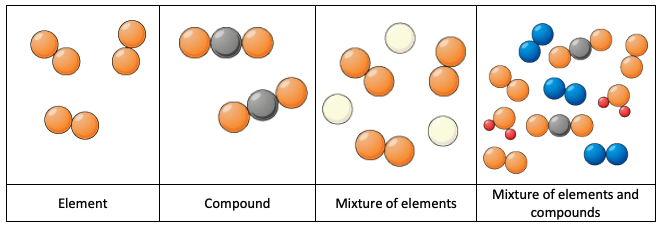
- Element: A substance made up of only one type of atom.
- Compound: A substance where atoms of different elements are chemically joined.
- Mixture of Elements: A physical combination of different elements, not chemically bonded.
- Mixture of Elements and Compounds: A physical combination of both elements and compounds.
Separation of Mixtures:
Atoms/molecules in mixtures can be easily separated by physical processes such as:
- Filtration
- Evaporation
- Chromatography
- Distillation
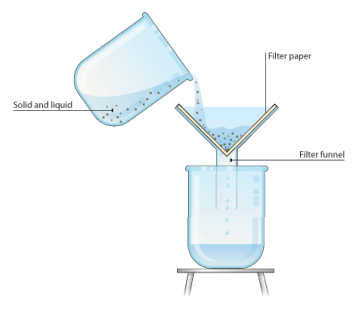
1. Filtration
Insoluble Solid from Liquid
Filtration is used to separate an insoluble solid from a liquid. (An insoluble substance is one that does not dissolve).
Example:
Sand can be separated from a mixture of sand and water using filtration. This is because sand does not dissolve in water.
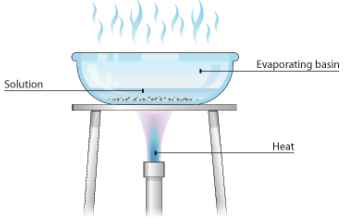
2. Evaporation
Soluble Solid from Liquid
Evaporation is used to separate a soluble solid from a liquid (a soluble substance does dissolve to form a solution).
Example:
Copper sulfate crystals can be separated from a copper sulfate solution using evaporation. It's important to remember that it is the water that evaporates away, not the solution itself.
3. Chromatography
Separating Liquids Due to Mass
Chromatography can be used to separate mixtures of coloured compounds. Mixtures that are suitable for separation by chromatography include inks, dyes, and colouring agents in food.
Process:
- Simple chromatography is typically carried out on paper.
- A spot of the mixture is placed near the bottom of a piece of chromatography paper.
- The paper is then placed upright in a suitable solvent, e.g., water.
- As the solvent soaks up the paper, it carries the mixture with it. Different components of the mixture move at different rates, effectively separating the mixture.
Values:
Different chromatograms and the separated components of the mixtures can be identified by calculating the value using the equation:
The value of a particular compound is always the same if the chromatography is carried out in the same way. This consistency allows industries to use chromatography to identify compounds in mixtures.
Example:
- In the diagram above, the blue dot has risen 4 cm, and the solvent has risen 10 cm. Therefore, the value of the blue dot is:
Practice: Try working out the values of the blue, purple, and yellow compounds here:
Distillation
Separating Liquids Due to Boiling Point
Distillation is used for separating water and miscible liquids.
- Pure liquids have specific boiling points. For example, water boils at 100°C and ethanol boils at 78°C.
- Water and ethanol are miscible (they mix together easily without separating into layers).
How It Works:
- This method works because the liquids in the mixture have different boiling points. When the mixture is heated, one liquid evaporates before the other.
500K+ Students Use These Powerful Tools to Master Mixtures For their GCSE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
110 flashcards
Flashcards on Mixtures
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards11 quizzes
Quizzes on Mixtures
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes29 questions
Exam questions on Mixtures
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Mixtures
Create custom exams across topics for better practice!
Try Chemistry exam builder26 papers
Past Papers on Mixtures
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Mixtures you should explore
Discover More Revision Notes Related to Mixtures to Deepen Your Understanding and Improve Your Mastery
