Photo AI
Last Updated Sep 26, 2025
Cells & Batteries Simplified Revision Notes for GCSE AQA Chemistry
Revision notes with simplified explanations to understand Cells & Batteries quickly and effectively.
342+ students studying
5.2.1 Cells & Batteries
Electrochemical Cells and Voltage
Electrochemical cells are devices that convert chemical energy into electrical energy. Unlike electrolysis, where a voltage is applied to cause chemical reactions, electrochemical cells generate voltage through chemical reactions. These cells are the fundamental units of batteries, and they consist of two main components: electrodes and an electrolyte.
-
Electrodes: Electrochemical cells have two electrodes, typically made of metals like copper, zinc, or platinum. One electrode is negative, and the other is positive. These electrodes are crucial because they conduct electricity and participate in the chemical reactions that produce voltage.
-
Electrolyte: The electrodes are immersed in an electrolyte, which is a solution containing ions. The electrolyte facilitates the flow of ions between the electrodes, which is essential for the chemical reactions that generate electricity.
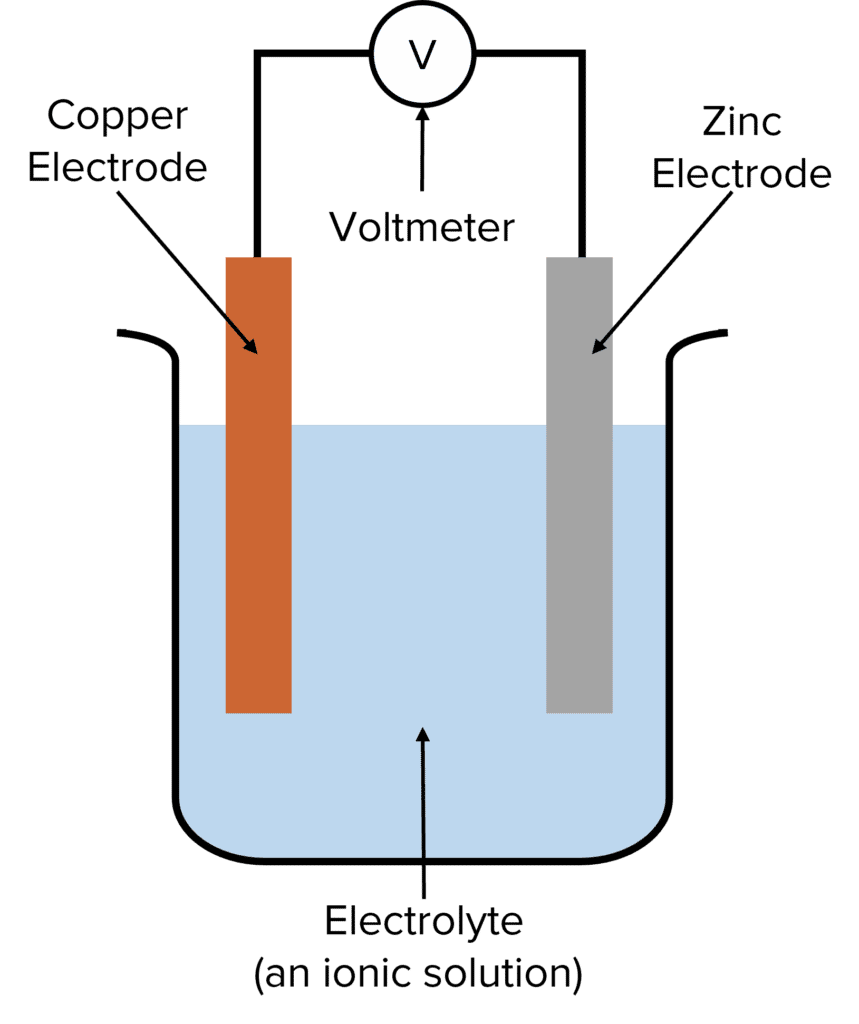
How Electrochemical Cells Generate Voltage
The chemical reactions at the electrodes create a difference in charge between them. This charge difference generates an electric current when the electrodes are connected by a wire. The voltage produced by an electrochemical cell can be measured using a voltmeter connected to this wire.
-
Voltage in a Single Cell: The voltage of an individual electrochemical cell is typically small, usually between 2 and 3 volts (V).
-
Connecting Cells in Series: To obtain a higher voltage, multiple cells can be connected in series, forming a battery. When connected in series, the total voltage of the battery is the sum of the voltages of the individual cells.
Example: If three cells have voltages of 2.3 V, 0.7 V, and 1.5 V respectively, the total voltage of the battery would be:
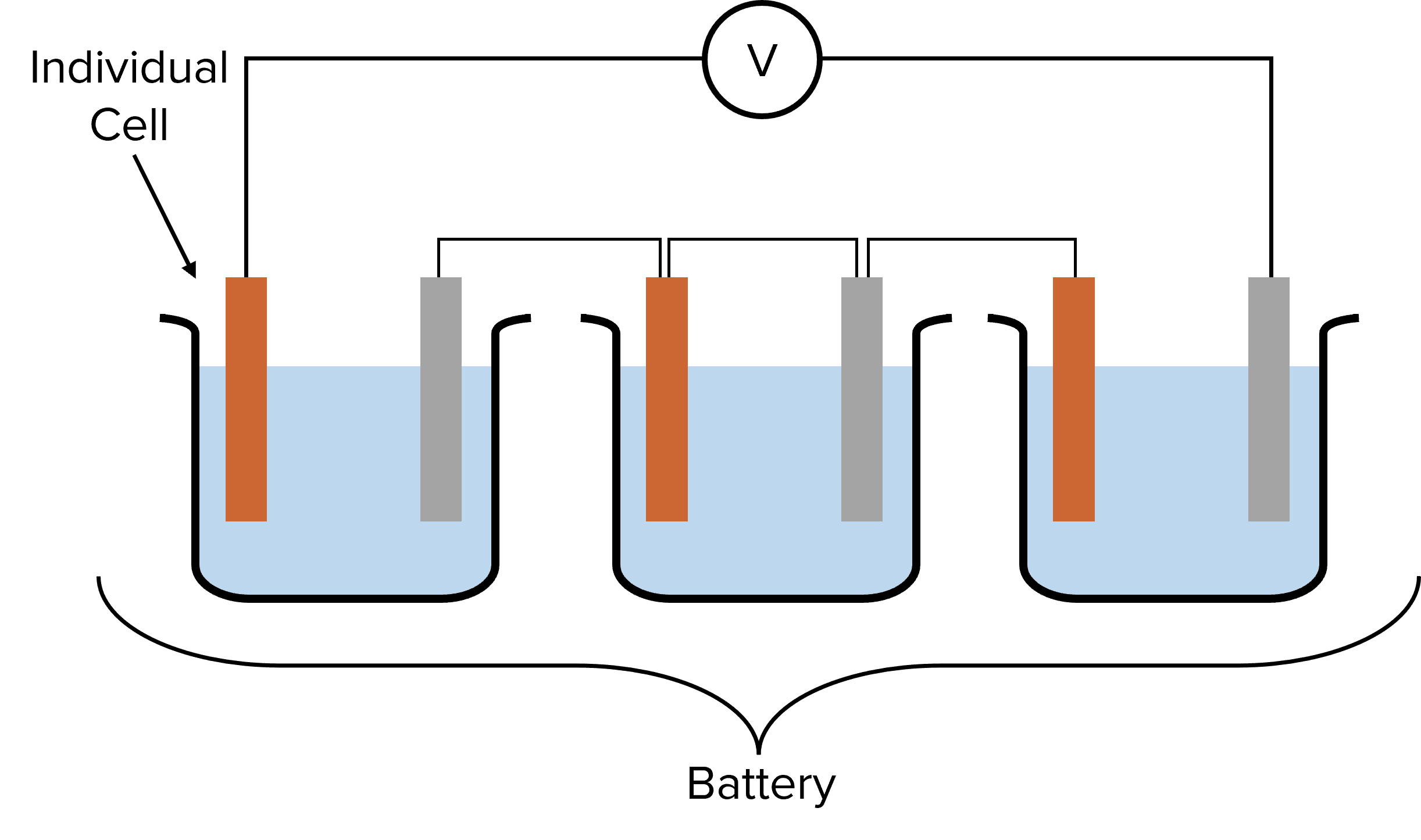
Factors Affecting Voltage
-
Reactivity of Metals: The reactivity difference between the metals used as electrodes affects the voltage. Greater differences in reactivity lead to higher voltages.
-
Type of Electrolyte: The electrolyte used in the cell also influences the voltage, as different ions react differently with the electrodes.
Rechargeable and Non-Rechargeable Batteries
Batteries made from electrochemical cells can be either rechargeable or non-rechargeable, depending on whether the chemical reactions within the cells are reversible or irreversible.
- Non-Rechargeable Batteries: In non-rechargeable batteries (e.g., alkaline batteries), the chemical reactions at the electrodes are irreversible. Once the reactants are used up, the battery can no longer produce electricity and must be replaced.
- Rechargeable Batteries: In rechargeable batteries (e.g., lithium-ion batteries), the chemical reactions can be reversed by applying an external voltage. This process regenerates the original reactants, allowing the battery to produce electricity again. This process is known as recharging.

Applications:
-
Non-rechargeable batteries: Commonly used in devices like remote controls and toys.
-
Rechargeable Batteries: Widely used in portable electronics, such as smartphones, tablets, and electric vehicles.
500K+ Students Use These Powerful Tools to Master Cells & Batteries For their GCSE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
30 flashcards
Flashcards on Cells & Batteries
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards3 quizzes
Quizzes on Cells & Batteries
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes29 questions
Exam questions on Cells & Batteries
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Cells & Batteries
Create custom exams across topics for better practice!
Try Chemistry exam builder26 papers
Past Papers on Cells & Batteries
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Cells & Batteries you should explore
Discover More Revision Notes Related to Cells & Batteries to Deepen Your Understanding and Improve Your Mastery
