Photo AI
Last Updated Sep 26, 2025
Fuel Cells Simplified Revision Notes for GCSE AQA Chemistry
Revision notes with simplified explanations to understand Fuel Cells quickly and effectively.
434+ students studying
5.2.2 Fuel Cells
Fuel cells are a special type of electrochemical cell that continuously produces electricity as long as they are supplied with fuel and an oxidising agent. Unlike traditional batteries that store chemical energy internally, fuel cells require a constant supply of fuel from an external source.
One of the most commonly used fuel cells is the hydrogen-oxygen fuel cell. In this cell, hydrogen gas (H₂) reacts with oxygen gas (O₂) to produce water (H₂O), generating electricity in the process.
How Hydrogen-Oxygen Fuel Cells Work The overall chemical reaction in a hydrogen-oxygen fuel cell is:
This reaction can be broken down into two redox half-equations that occur at the anode and cathode of the fuel cell:
- Anode Reaction (Oxidation):
- Hydrogen gas (H₂) is fed into the fuel cell at the anode.
- The hydrogen molecules are oxidised, losing electrons and forming hydrogen ions (H⁺):
- The electrons released travel through an external circuit, creating an electric current that can be used to power devices.
- Cathode Reaction (Reduction):
- Oxygen gas (O₂) is introduced at the cathode.
- The oxygen molecules gain the electrons coming from the external circuit and react with the hydrogen ions (H⁺) to form water:
These reactions occur simultaneously, with the electrons flowing from the anode to the cathode through the external circuit, and the ions moving through the electrolyte within the cell.
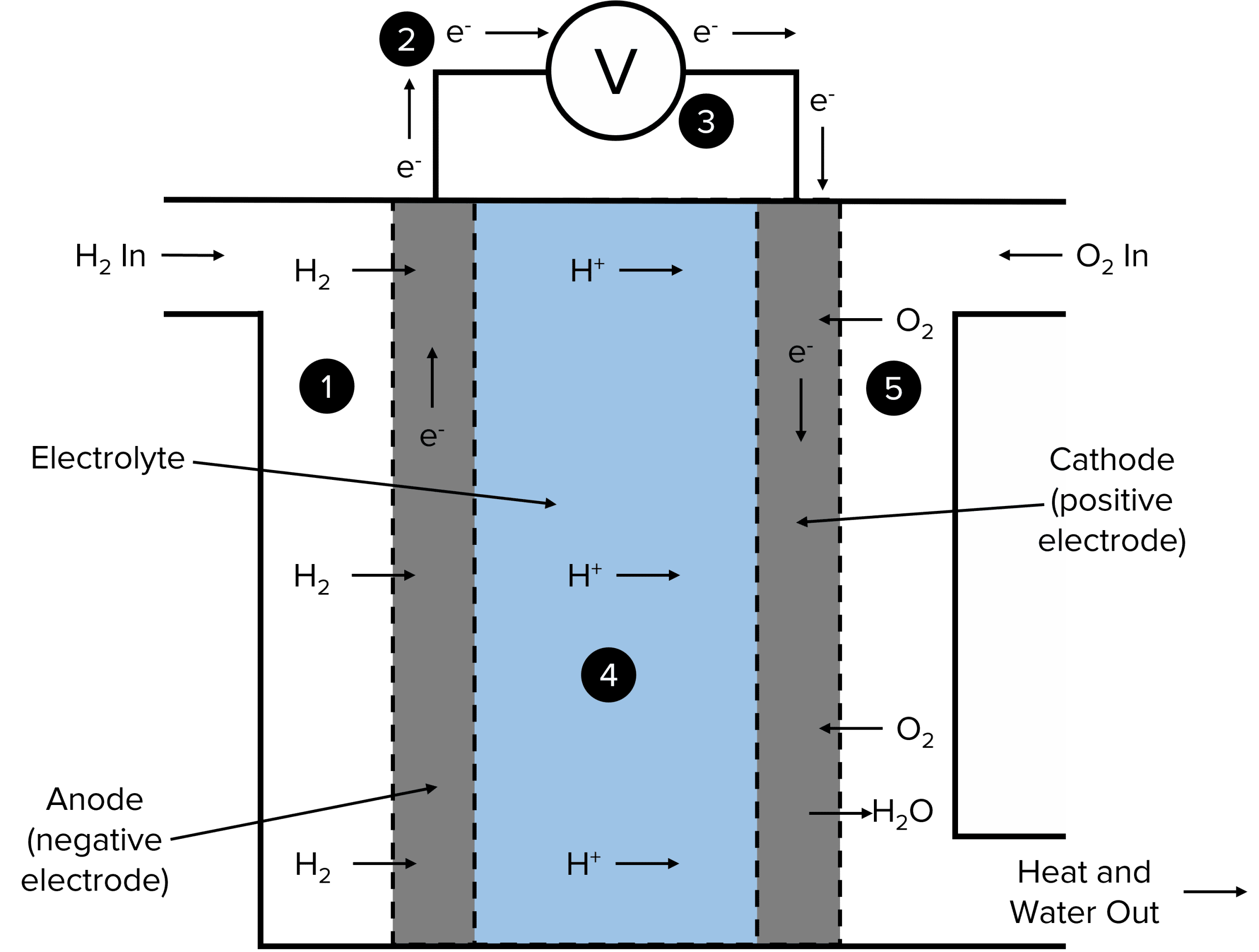
Advantages of Hydrogen-Oxygen Fuel Cells
- Environmental Impact: Hydrogen-oxygen fuel cells are seen as environmentally friendly because their only by-product is water, which is non-polluting. This contrasts with traditional batteries, which may contain harmful chemicals.
- Sustainability: Since fuel cells do not require the materials to be replaced often, they generate less waste compared to non-rechargeable batteries. Oxygen is readily available from the air, and hydrogen can be produced from water, making the fuel sources relatively accessible.
Challenges of Hydrogen-Oxygen Fuel Cells
- Hydrogen Storage: Hydrogen is a highly flammable gas and requires careful storage. It also takes up more space than liquid or solid fuels, which presents challenges in transportation and storage.
- Infrastructure and Cost: Developing the infrastructure needed to produce, store, and distribute hydrogen is still expensive and complex.
Applications of Hydrogen-Oxygen Fuel Cells
- Spacecraft: Hydrogen-oxygen fuel cells have been used as a reliable power source in spacecraft, such as the Space Shuttle and the International Space Station.
- Potential for Electric Vehicles: There is growing interest in using hydrogen-oxygen fuel cells to power electric vehicles due to their efficiency and low environmental impact.
500K+ Students Use These Powerful Tools to Master Fuel Cells For their GCSE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
30 flashcards
Flashcards on Fuel Cells
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards3 quizzes
Quizzes on Fuel Cells
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes29 questions
Exam questions on Fuel Cells
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Fuel Cells
Create custom exams across topics for better practice!
Try Chemistry exam builder26 papers
Past Papers on Fuel Cells
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Fuel Cells you should explore
Discover More Revision Notes Related to Fuel Cells to Deepen Your Understanding and Improve Your Mastery
