Photo AI
Last Updated Sep 26, 2025
Crude Oil, Hydrocarbons & Alkanes Simplified Revision Notes for GCSE AQA Chemistry
Revision notes with simplified explanations to understand Crude Oil, Hydrocarbons & Alkanes quickly and effectively.
326+ students studying
7.1.1 Crude Oil, Hydrocarbons & Alkanes
Hydrocarbons
Hydrocarbons are compounds that contain only hydrogen and carbon atoms. These atoms are joined together in chains and rings. Hydrocarbons can be either saturated or unsaturated.
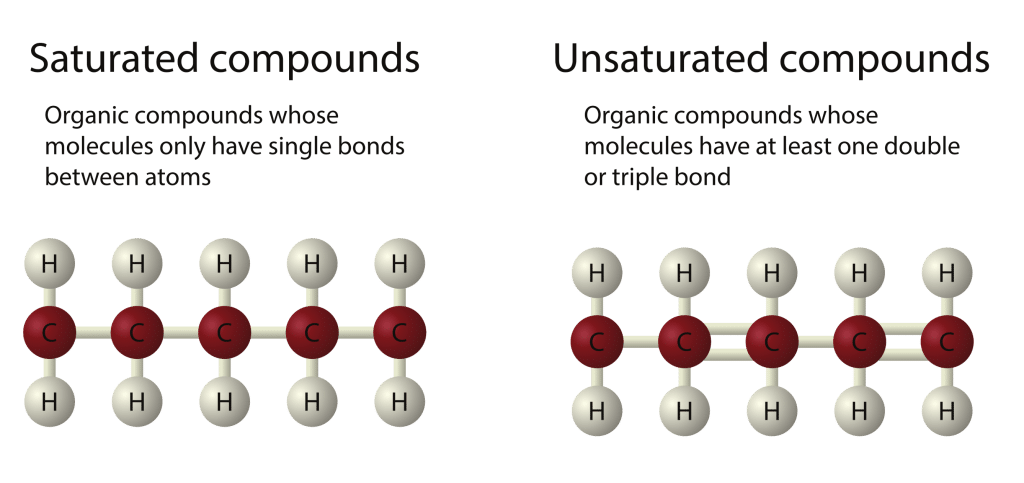
- In this image, the carbon atoms are red, and the hydrogen atoms are grey
Homologous series
To better understand hydrocarbons, scientists have organised them into groups called homologous series. A homologous series is a family of hydrocarbons that have similar properties and share the same general formula. This means that chemically, they all behave in the same way and have similar reactions, including having the same functional group of atoms.
However, hydrocarbons in a homologous series show a gradual variation in physical properties, such as melting and boiling points.
For example, as the number of carbon atoms in a hydrocarbon increases, so does its boiling point. This means that hydrocarbons with longer carbon chains have higher boiling points than those with shorter chains.
Crude Oil
Crude oil (also known as petroleum) is a mixture of different hydrocarbons found deep underground. It naturally formed over millions of years from the remains of dead plants and animals, through a process that involved high heat and pressure.
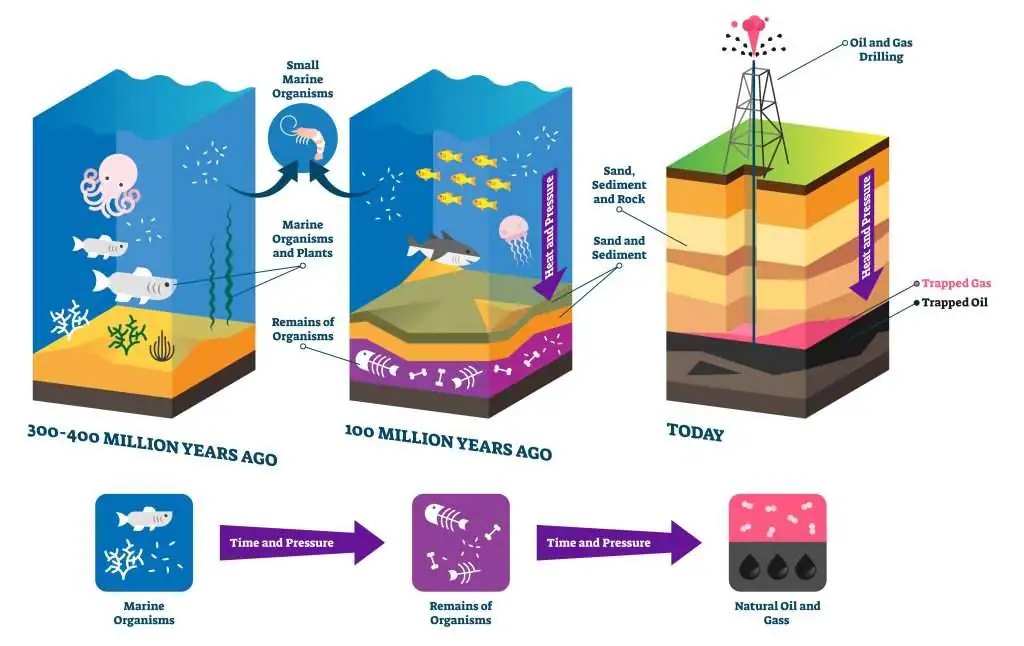
The appearance of crude oil can vary but it usually appears as a liquid, and we extract it by drilling into the ground. After extraction, the different hydrocarbons in the oil can be separated. It's important to remember that crude oil is a finite (limited) resource. It took millions of years to form, but we are using it much faster than it can be naturally replaced. If we keep burning it for energy, it will eventually run out.
Alkanes
Alkanes are a homologous series of saturated hydrocarbons made up of only carbon and hydrogen atoms. "Saturated" means they have the maximum number of hydrogen atoms possible, with only single bonds between the carbon atoms — no double bonds.
The general formula for alkanes is:
The letter 'n' represents the number of carbon atoms in an alkane molecule. As the number of carbon atoms increases in an alkane chain, its physical properties change, such as a gradual increase in boiling points.
Even though their physical properties vary, alkanes have similar chemical properties. They can burn in combustion, react with halogens, and can also be broken down into smaller molecules through a process called cracking.
500K+ Students Use These Powerful Tools to Master Crude Oil, Hydrocarbons & Alkanes For their GCSE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
50 flashcards
Flashcards on Crude Oil, Hydrocarbons & Alkanes
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards5 quizzes
Quizzes on Crude Oil, Hydrocarbons & Alkanes
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes29 questions
Exam questions on Crude Oil, Hydrocarbons & Alkanes
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Crude Oil, Hydrocarbons & Alkanes
Create custom exams across topics for better practice!
Try Chemistry exam builder26 papers
Past Papers on Crude Oil, Hydrocarbons & Alkanes
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Crude Oil, Hydrocarbons & Alkanes you should explore
Discover More Revision Notes Related to Crude Oil, Hydrocarbons & Alkanes to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Hydrocarbons: Fuel & Feedstock
Fractional Distillation & Petrochemicals
280+ studying
194KViews