Photo AI
Last Updated Sep 27, 2025
Elements, Compounds and Mixtures Simplified Revision Notes for Junior Cycle Science
Revision notes with simplified explanations to understand Elements, Compounds and Mixtures quickly and effectively.
434+ students studying
Elements, Compounds and Mixtures
Elements
Element: a substance that cannot be broken down into simpler substances by chemical means.
-
Over 100 different elements have been discovered.
- These elements are organised in the Periodic Table of Elements.
- Some symbols are derived from their Latin names, such as Na for sodium (Natrium).
- The first letter is always capitalised; if there is a second letter, it is lowercase (e.g., Helium = He).
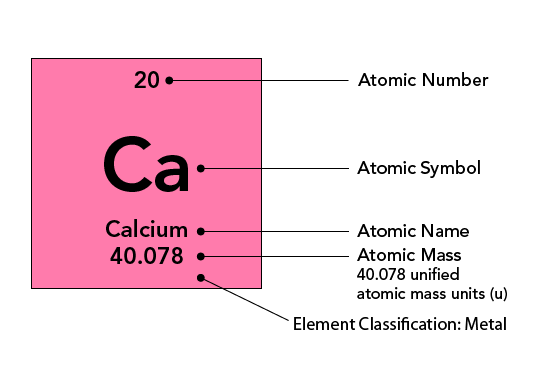
-
Each element has a symbol, usually one or two letters, typically derived from its name.
Robert Boyle
Robert Boyle, an Irish scientist from Lismore, Co. Waterford, defined the concept of an element.

Compounds
Compound: two or more different elements combined together chemically.
- Compounds consist of two or more different elements chemically combined.eg, MgO, H2O, FeS, and NaCl.
- When elements combine, their separate properties are lost and they gain new properties.
- Generally, elements are combined and heated to chemically bond. HYDROGEN gas can be combined with OXYGEN to make WATER (H₂O)
| Formula | Element | Description |
|---|---|---|
| H₂O | Hydrogen | An explosive gas |
| Oxygen | A highly flammable gas | |
| Water | A liquid | |
| CO₂ | Carbon | A black sooty solid |
| Oxygen | A highly flammable gas | |
| Carbon dioxide | A gas that puts out fires |
Mixtures
Mixture: consists of two or more substances mingled together but not chemically combined.
- Examples of mixtures include air, seawater, crude oil, tea
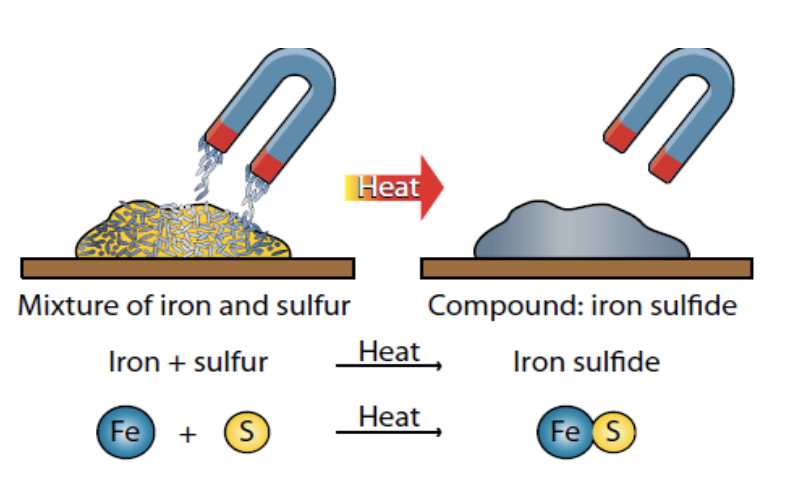
- Iron and sulfur mixed together form a mixture.
- Mixtures are easy to separate.

Differences Between Mixtures and Compounds
| Mixture | Compound |
|---|---|
| Properties are similar to the elements within | Properties are different from the elements |
| Contains two or more substances | One substance |
| Mostly easy to separate the substances | Very difficult to separate the elements |
Iron and Sulfur Experiment
This experiment demonstrates the difference between mixtures and compounds.
500K+ Students Use These Powerful Tools to Master Elements, Compounds and Mixtures For their Junior Cycle Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
40 flashcards
Flashcards on Elements, Compounds and Mixtures
Revise key concepts with interactive flashcards.
Try Science Flashcards4 quizzes
Quizzes on Elements, Compounds and Mixtures
Test your knowledge with fun and engaging quizzes.
Try Science Quizzes29 questions
Exam questions on Elements, Compounds and Mixtures
Boost your confidence with real exam questions.
Try Science Questions27 exams created
Exam Builder on Elements, Compounds and Mixtures
Create custom exams across topics for better practice!
Try Science exam builder30 papers
Past Papers on Elements, Compounds and Mixtures
Practice past papers to reinforce exam experience.
Try Science Past PapersOther Revision Notes related to Elements, Compounds and Mixtures you should explore
Discover More Revision Notes Related to Elements, Compounds and Mixtures to Deepen Your Understanding and Improve Your Mastery
Load more notes