Photo AI
Last Updated Sep 27, 2025
Solutions and Formation of Crystals Simplified Revision Notes for Junior Cycle Science
Revision notes with simplified explanations to understand Solutions and Formation of Crystals quickly and effectively.
456+ students studying
Solutions and Formation of Crystals
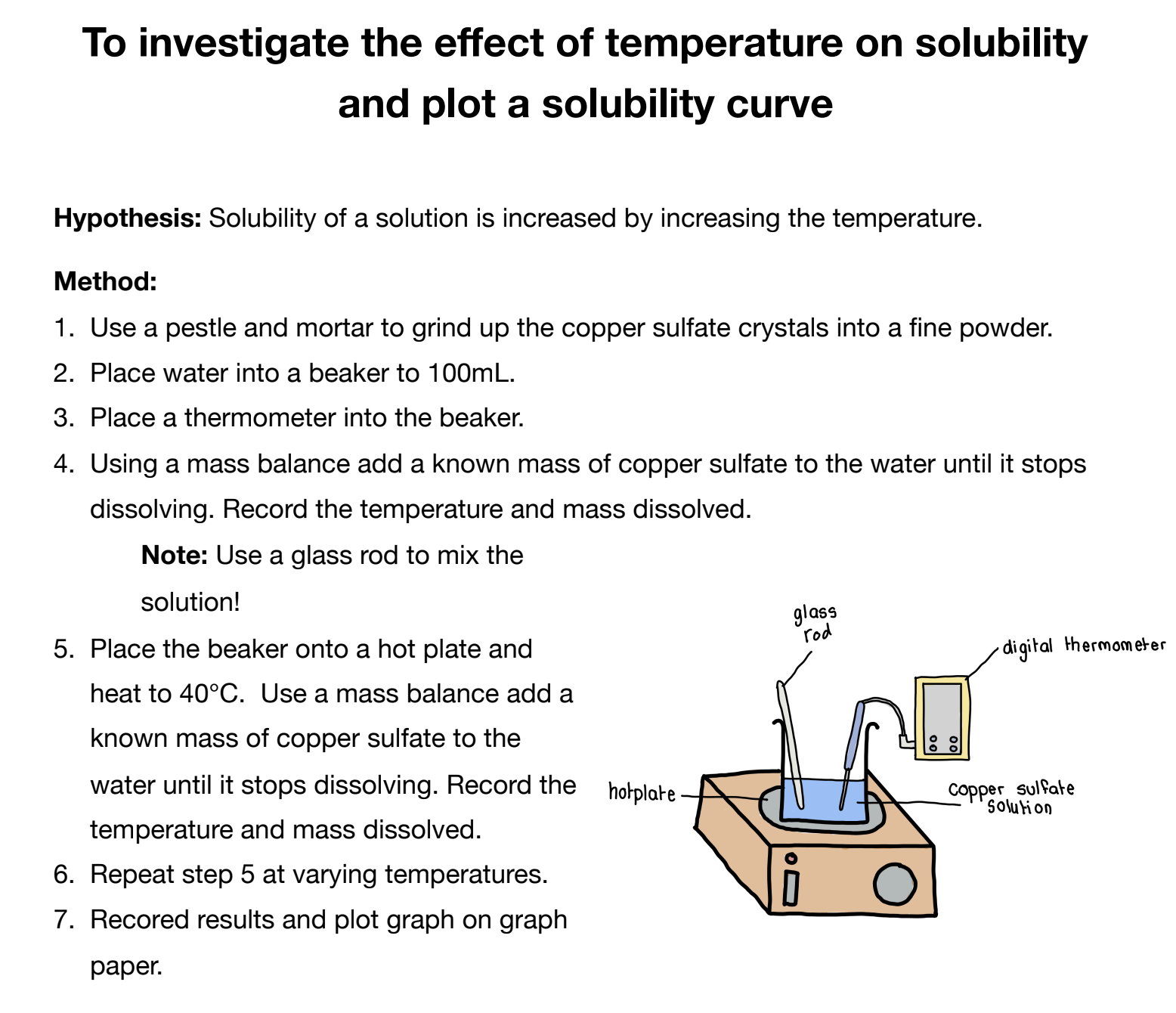
A solute dissolves in a solvent to form a solution. Solute**+Solvent=**Solution
Salt+Water=Saltwater solution
Solvent
Solvent: A substance that dissolves other materials to form a solution.
Water and sugar: Water dissolves sugar, so it is a solvent.
Water and plastic: Water does not dissolve plastic, so it is not a solvent in this case.
Other Solvents:
- Hydrochloric Acid (HCl): Dissolves food in the stomach.
- Ethanol: Dissolves ink.

Solute
Solute: A substance that dissolves in the solvent.
- Sugar in Water: Sugar is the solute when it dissolves in water.
- Food in HCl and Blood Plasma: Food particles are solutes in both hydrochloric acid and blood plasma.
- Insoluble Substances: If a substance does not dissolve in a solvent, it is insoluble. For example, plastic is insoluble in water.
- Gaseous Solutes: A solute can also be a gas. For example, oxygen dissolved in water is essential for fish.
Solution
Solution: Solute dissolved in a solvent.
Sand and Water: Sand and water is not a solution because sand does not dissolve (is insoluble) in water.
Salt and Water: Salt and water is a solution as salt dissolves (is soluble) in water.
Concentrated and Dilute Solutions
- Dilute Solution: Contains a small amount of solute in a large amount of solvent.
- Concentrated Solution: Contains a large amount of solute in a small amount of solvent.
- Colour Intensity: A more intense colour indicates a higher concentration.
Solubility
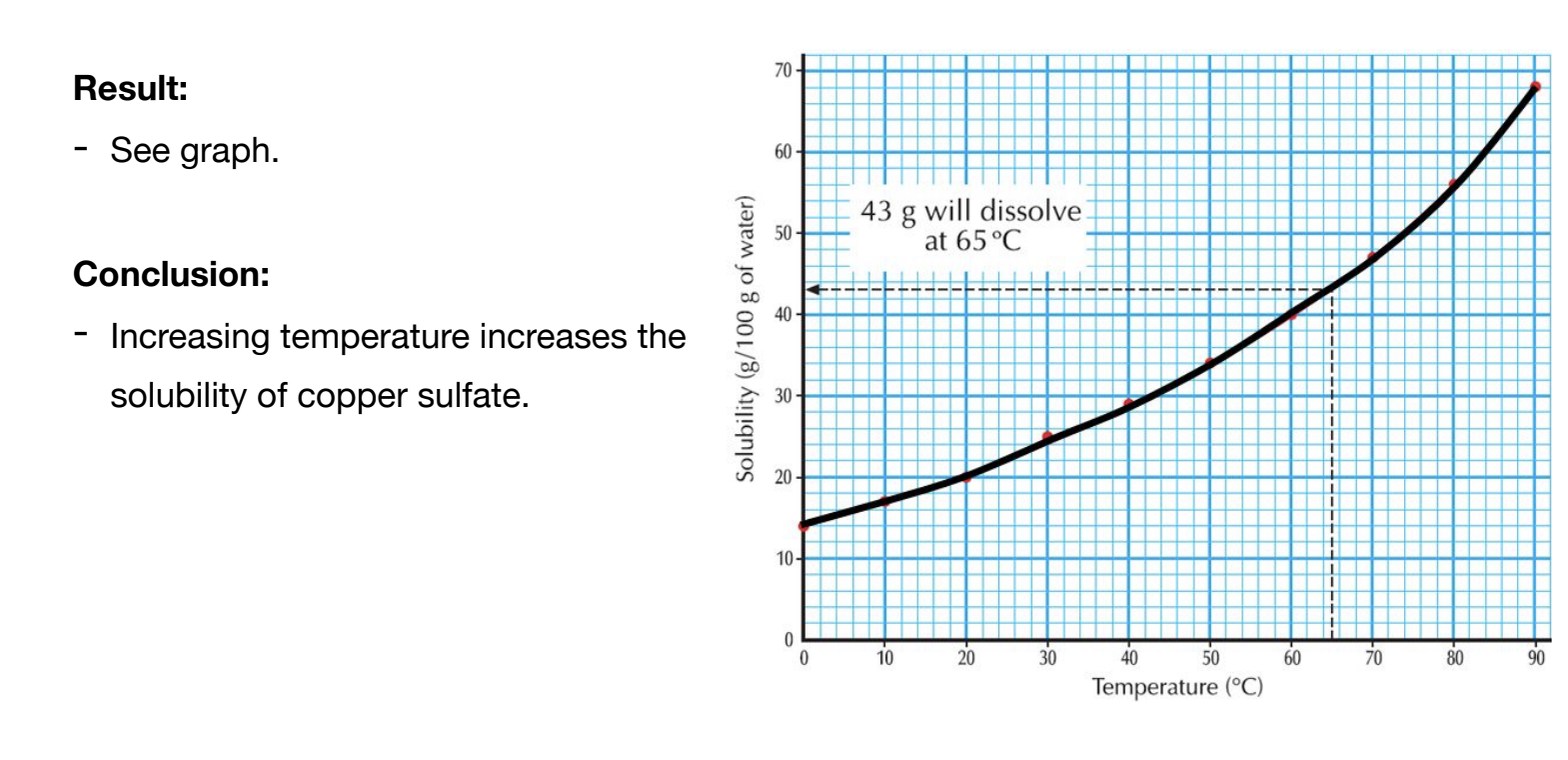
Solubility curves are used to plot the relationship between temperature and solubility of a solute.
Temperature: Solubility increases with temperature.
Drawing Solubility Graphs: Do not use a ruler to connect the dots; draw freehand.
Increasing Solubility
- Increase the temperature.
- Stir the solution to help the solute dissolve faster.
- Add more solvent.
Determining solubility at 65°C using a graph:
- Draw a vertical line at 65°C until it meets the curve.
- Draw a horizontal line to the Y-axis to find the solubility.
Exam Tip! Make sure you show evidence on your graph of how you found your answer. eg, draw dashed lines
:::
Crystallisation
Crystallisation: the forming of crystals by the cooling of a saturated solution.
Saturated solution: Contains as much dissolved solute as possible at a given temperature.
Crystal Formation Process
- Dissolve solute: Dissolve solute in water until no more can dissolve at that temperature.
- Increase temperature: Dissolve more solute to make the solution saturated.
- Cool down: Turn off the heat and place the solution in a fridge for 24 hours.
- Result: Crystals form at the bottom of the container.
Cooling and Crystal Size
- Cooled quickly by ice: Small crystals.
- Cooled slowly: Large crystals.
Summary note: If one substance does not dissolve in another, it is insoluble.
If one substance does dissolve in another, it is soluble.
A mixture of an insoluble substance in another substance is called a suspension.
Mandatory Experiment
500K+ Students Use These Powerful Tools to Master Solutions and Formation of Crystals For their Junior Cycle Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
40 flashcards
Flashcards on Solutions and Formation of Crystals
Revise key concepts with interactive flashcards.
Try Science Flashcards4 quizzes
Quizzes on Solutions and Formation of Crystals
Test your knowledge with fun and engaging quizzes.
Try Science Quizzes29 questions
Exam questions on Solutions and Formation of Crystals
Boost your confidence with real exam questions.
Try Science Questions27 exams created
Exam Builder on Solutions and Formation of Crystals
Create custom exams across topics for better practice!
Try Science exam builder30 papers
Past Papers on Solutions and Formation of Crystals
Practice past papers to reinforce exam experience.
Try Science Past PapersOther Revision Notes related to Solutions and Formation of Crystals you should explore
Discover More Revision Notes Related to Solutions and Formation of Crystals to Deepen Your Understanding and Improve Your Mastery
Load more notes