Photo AI
Last Updated Sep 27, 2025
Periodic Trends: Atomic Radii and Ionisation Energies Simplified Revision Notes for Leaving Cert Chemistry
Revision notes with simplified explanations to understand Periodic Trends: Atomic Radii and Ionisation Energies quickly and effectively.
444+ students studying
Periodic Trends: Atomic Radii and Ionisation Energies
Atomic Radii (Covalent Radii Only)
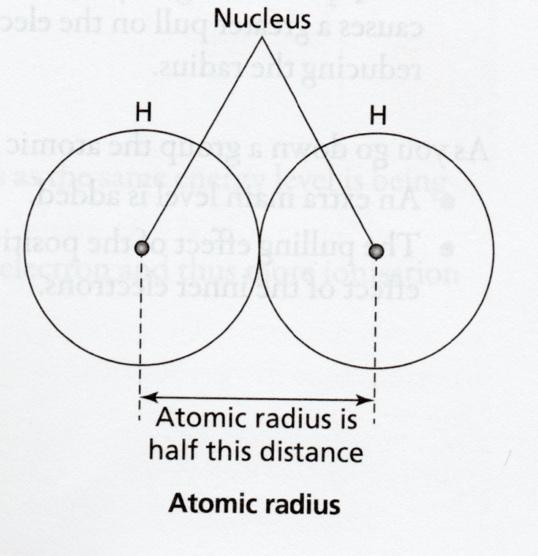
Atomic radius
Atomic radius is half the distance between the centres of neighbouring atoms which are joined together by a single covalent bond. For example, the atomic radius of hydrogen is half the distance between the nuclei of the two hydrogen nuclei which are covalently bonded.
Covalent radius
Covalent radius is the measure of the size of an atom, specifically half the distance between the nuclei of two identical atoms joined by a covalent bond.
Trends in Atomic Radii:
Down a Group:
- As you move down a group in the periodic table, atomic radii increase.
- Reason: Each successive element has an additional energy level (shell), which increases the distance between the nucleus and the outermost electrons. Despite the increasing nuclear charge (more protons), the shielding effect of inner electrons reduces the pull on the outer electrons, allowing the atom to expand.
Across a Period:
- As you move across a period from left to right, atomic radii decrease.
- Reason: The number of protons increases, leading to a greater nuclear charge. However, the electrons are being added to the same energy level, so there is no additional shielding. This increased nuclear attraction pulls the electrons closer to the nucleus, reducing the atomic radius.
First Ionisation Energies:
First ionisation energy is the energy required to remove the outermost electron from a neutral atom in its gaseous state.
Trends in First Ionisation Energies:
Down a Group:
- Ionisation energy decreases as you move down a group.
- Reason: The outer electrons are farther from the nucleus due to the increased number of energy levels. The shielding effect of inner electrons weakens the attraction between the nucleus and the outer electrons, making it easier to remove an electron.
Across a Period:
- Ionisation energy increases across a period from left to right.
- Reason: The nuclear charge increases as protons are added, pulling the outer electrons closer to the nucleus and making them harder to remove. The increased attraction between the nucleus and the outer electrons raises the ionisation energy.
Exceptions:
- Group 13 (e.g., Boron): Ionisation energy is slightly lower than expected because the outer electron is in a p-orbital, which is slightly higher in energy than the s-orbital, making it easier to remove.
- Group 16 (e.g., Oxygen): Ionisation energy is slightly lower than expected due to electron-electron repulsion in the doubly occupied p-orbital, making it easier to remove one of the paired electrons.
Second and Successive Ionisation Energies:
- Second ionisation energy is the energy required to remove an additional electron after the first electron has been removed. Successive ionisation energies refer to the energies required to remove further electrons.
- Trend: Each successive ionisation energy is higher than the previous one. This is because removing an electron decreases the electron-electron repulsion, pulling the remaining electrons closer to the nucleus. Additionally, after the first electron is removed, the resulting ion has a greater effective nuclear charge (more protons than electrons), making it more difficult to remove subsequent electrons.
Evidence for Energy Levels Provided by Successive Ionisation Energies:
- Sharp increases in ionisation energy provide evidence for energy levels. When an electron is removed from a filled inner shell (closer to the nucleus), the ionisation energy increases dramatically.
- For example, the first and second ionisation energies of sodium (Na) are relatively low because they remove electrons from the outermost shell. However, the third ionisation energy is extremely high because it involves removing an electron from a full inner shell (closer to the nucleus), which requires much more energy.
Exam Tip: Be ready to explain trends in both atomic radii and ionisation energies using concepts like nuclear charge, shielding effect, and electron configuration. Pay attention to exceptions, such as in Groups 13 and 16 for ionisation energies, and understand how successive ionisation energies provide evidence for the existence of distinct energy levels.
Dependence of Chemical Properties of Elements on Their Electronic Structure
The chemical properties of elements are heavily influenced by their electronic structure, particularly the arrangement of electrons in their outermost energy levels (valence electrons). Key factors such as atomic radius, nuclear charge, and the screening effect help explain trends in the reactivity and chemical behaviour of elements, especially in Groups I (Alkali Metals) and Group VII (Halogens).
Group I: Alkali Metals (e.g., , , )
Electronic Structure and Chemical Properties:
- Electronic Structure: All alkali metals have a single electron in their outermost shell (e.g., ). This lone electron is easily lost in chemical reactions, making these metals highly reactive and prone to forming +1 ions (e.g., ).
- Reactivity: Reactivity increases as you move down the group. This trend can be explained by considering the atomic radius, nuclear charge, and screening effect.
Explanations for Trends in Reactivity:
- Atomic Radius:
- As you move down Group I, the atomic radius increases because each successive element has an additional energy level.
- The larger distance between the nucleus and the outermost electron makes it easier to remove the electron, increasing reactivity.
- Screening Effect:
- The inner electrons shield the outermost electron from the full attraction of the nucleus.
- As the number of inner electron shells increases down the group, the screening effect increases.
- This weakens the attraction between the nucleus and the outer electron, making it easier for the atom to lose the electron, thus increasing reactivity.
- Nuclear Charge:
- Although the nuclear charge (the number of protons) increases as you move down the group, the effect of this is offset by the increasing atomic radius and the greater screening by inner electrons.
- As a result, the outermost electron experiences less attraction, making it easier to lose.
Summary for Group I:
- Reactivity increases down the group due to the increasing atomic radius, greater screening effect, and weaker effective nuclear charge on the outermost electron.
- This makes it easier for Group I elements to lose their single valence electron in reactions, forming positive ions.
Group VII: Halogens (e.g., , , , )
Electronic Structure and Chemical Properties:
- Electronic Structure: Halogens have seven electrons in their outermost shell (e.g., ).
- They typically gain one electron to achieve a full outer shell, making them highly reactive and prone to forming -1 ions (e.g., ).
- Reactivity: Reactivity decreases as you move down the group.
- This trend is also explained by atomic radius, screening effect, and nuclear charge.
Explanations for Trends in Reactivity:
- Atomic Radius:
- As you move down Group VII, the atomic radius increases due to additional energy levels.
- The outermost electrons are farther from the nucleus, making it more difficult for the atom to attract and gain an additional electron.
- This leads to a decrease in reactivity as you move down the group.
- Screening Effect:
- The screening effect increases as more inner electron shells are added down the group.
- This shields the outermost electrons from the full nuclear attraction, making it harder for the halogen atom to attract an electron from another atom.
- This weakening of the nuclear pull contributes to the decrease in reactivity.
- Nuclear Charge:
- The nuclear charge increases as you move down the group (more protons in the nucleus), but the increased distance of the outer electrons and the stronger screening effect reduce the effective attraction between the nucleus and the incoming electron.
- This results in a lower tendency for halogens to gain electrons as you go down the group.
Summary for Group VII:
- Reactivity decreases down the group due to the increasing atomic radius, stronger screening effect, and weaker effective nuclear attraction on the outermost electrons.
- This makes it harder for Group VII elements to attract and gain an electron, reducing their reactivity.
Exam Tip: Be prepared to explain how atomic radius, screening effect, and nuclear charge affect the reactivity trends in Groups I and VII. Understand the relationship between electronic structure and chemical properties and how this leads to different trends in losing or gaining electrons in these groups.
500K+ Students Use These Powerful Tools to Master Periodic Trends: Atomic Radii and Ionisation Energies For their Leaving Cert Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
180 flashcards
Flashcards on Periodic Trends: Atomic Radii and Ionisation Energies
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards18 quizzes
Quizzes on Periodic Trends: Atomic Radii and Ionisation Energies
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes29 questions
Exam questions on Periodic Trends: Atomic Radii and Ionisation Energies
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Periodic Trends: Atomic Radii and Ionisation Energies
Create custom exams across topics for better practice!
Try Chemistry exam builder115 papers
Past Papers on Periodic Trends: Atomic Radii and Ionisation Energies
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Periodic Trends: Atomic Radii and Ionisation Energies you should explore
Discover More Revision Notes Related to Periodic Trends: Atomic Radii and Ionisation Energies to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Atomic Structure
Energy Levels, Sublevels and Atomic Orbitals
498+ studying
189KViews