Photo AI
Last Updated Sep 27, 2025
Aromatic Hydrocarbons Simplified Revision Notes for Leaving Cert Chemistry
Revision notes with simplified explanations to understand Aromatic Hydrocarbons quickly and effectively.
375+ students studying
Aromatic Hydrocarbons
What are Aromatic Hydrocarbons?
Aromatic hydrocarbons, also known as arenes, are a class of hydrocarbons characterized by a ring structure with delocalized electrons. The most well-known example is benzene, which forms the basis of many aromatic compounds.
Structure of Aromatic Compounds
Benzene ()
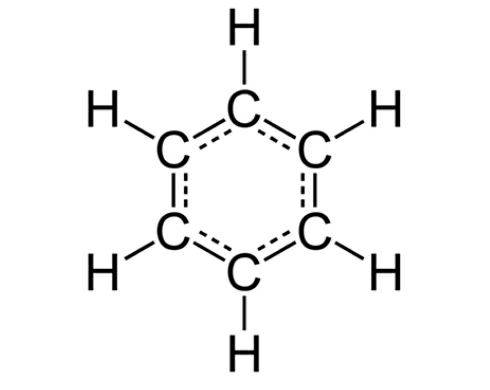
- Bonding: Benzene consists of six carbon atoms arranged in a hexagonal ring. Each carbon is bonded to one hydrogen atom.
- Delocalization: The six pi-electrons in benzene are shared across the ring, creating a system of delocalized electrons. This delocalization provides benzene with extra stability.
- Representation: Instead of alternating single and double bonds, benzene is often represented as a hexagon with a circle inside, symbolizing the equal distribution of electrons.
Methylbenzene (Toluene, )
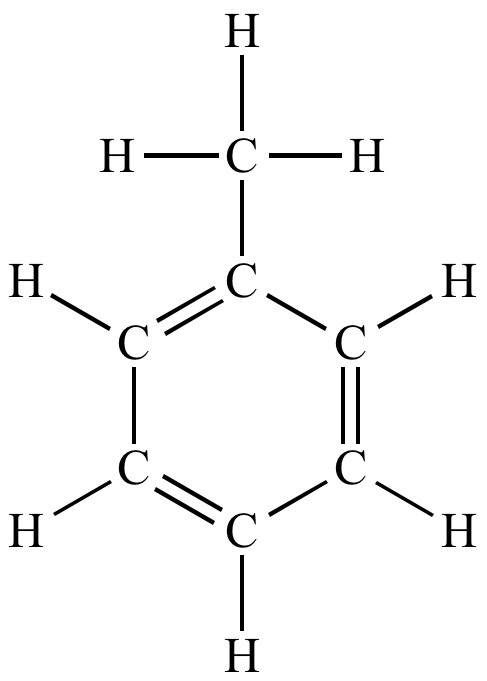
- Structure: Methylbenzene is a benzene ring with one hydrogen atom replaced by a methyl group ().
- Properties: Like benzene, methylbenzene is non-polar and has similar chemical behaviour.
Ethylbenzene ()
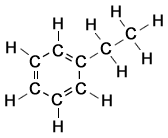
- Structure: Ethylbenzene consists of a benzene ring with an ethyl group () attached. It is an important intermediate in the production of styrene (used to make polystyrene plastics).
Physical Properties of Aromatic Hydrocarbons
Physical State: Benzene, methylbenzene, and ethylbenzene are liquids at room temperature.
Solubility:
- Non-polar: Aromatic hydrocarbons are non-polar molecules.
- Solubility in Water: They are insoluble in water due to their non-polar nature.
- Solubility in Non-Polar Solvents: They dissolve easily in non-polar solvents such as hexane or benzene itself.
Demonstration of Solubility of Methylbenzene (Toluene)
To demonstrate the solubility properties of methylbenzene:
- When mixed with water, methylbenzene does not dissolve and forms a separate layer, indicating that it is insoluble in polar solvents.
- However, when mixed with a non-polar solvent like hexane, methylbenzene dissolves readily, demonstrating its solubility in non-polar solvents.
Summary of Physical Properties
| Compound | Formula | State at Room Temperature | Solubility in Water | Solubility in Non-Polar Solvents |
|---|---|---|---|---|
| Benzene | Liquid | Insoluble | Soluble | |
| Methylbenzene | Liquid | Insoluble | Soluble | |
| Ethylbenzene | Liquid | Insoluble | Soluble |
500K+ Students Use These Powerful Tools to Master Aromatic Hydrocarbons For their Leaving Cert Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
120 flashcards
Flashcards on Aromatic Hydrocarbons
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards12 quizzes
Quizzes on Aromatic Hydrocarbons
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes29 questions
Exam questions on Aromatic Hydrocarbons
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Aromatic Hydrocarbons
Create custom exams across topics for better practice!
Try Chemistry exam builder115 papers
Past Papers on Aromatic Hydrocarbons
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Aromatic Hydrocarbons you should explore
Discover More Revision Notes Related to Aromatic Hydrocarbons to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Fuels and Heats of Reaction
Structure of Aliphatic Carbons
369+ studying
186KViews96%
114 rated
Fuels and Heats of Reaction
Exothermic and Endothermic Reactions
402+ studying
196KViews