Photo AI
Last Updated Sep 27, 2025
Catalysts Simplified Revision Notes for Leaving Cert Chemistry
Revision notes with simplified explanations to understand Catalysts quickly and effectively.
316+ students studying
Catalysts
What is a Catalyst?
A catalyst increases the rate of a reaction without being consumed in the process. It works by lowering the activation energy needed for the reaction.
Examples:
- Platinum catalyst: Speeds up the reaction between hydrogen and oxygen gases.
- Enzymes: Biological catalysts that increase the rate of reactions in living cells. Two examples include:
- Amylase: Breaks down starch into sugars.
- Catalase: Speeds up the decomposition of hydrogen peroxide into water and oxygen.
Catalytic Converters
Catalytic converters in cars use catalysts to speed up the reaction of harmful gases:
- Nature of catalysts: Typically platinum, palladium, or rhodium.
- Reactions catalyzed:
- Carbon monoxide is converted to carbon dioxide.
- Nitrogen oxides are reduced to nitrogen and oxygen.
- Environmental benefits: Reduces harmful emissions and improves air quality.
Catalyst Poisons:
Substances like lead can poison catalysts by binding to their surfaces and reducing their effectiveness.
Activation Energy and Temperature
- Activation energy is the minimum energy required for a reaction to occur. Catalysts lower this barrier, allowing the reaction to proceed faster.
- As temperature increases, more particles have enough energy to overcome the activation energy, increasing the reaction rate.
Reaction Profile Diagrams:
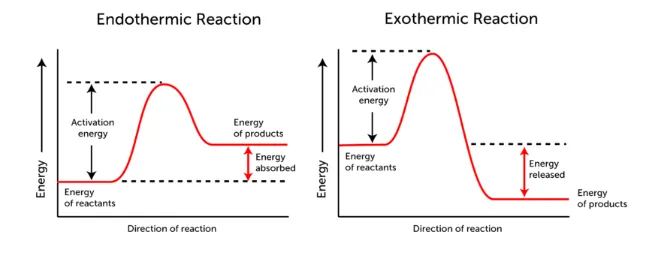
- Without a catalyst: The activation energy is higher.
- With catalyst: The activation energy is lower, and the reaction occurs more easily.
Demonstrations
- Oxidation of Methanol: When methanol is exposed to a hot platinum wire, it oxidizes rapidly, demonstrating the effect of a catalyst.
- Oxidation of Potassium Sodium Tartrate: Adding cobalt(II) salts speeds up the decomposition of potassium sodium tartrate by hydrogen peroxide, demonstrating the effect of a catalyst.
500K+ Students Use These Powerful Tools to Master Catalysts For their Leaving Cert Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
60 flashcards
Flashcards on Catalysts
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards6 quizzes
Quizzes on Catalysts
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes29 questions
Exam questions on Catalysts
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Catalysts
Create custom exams across topics for better practice!
Try Chemistry exam builder115 papers
Past Papers on Catalysts
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Catalysts you should explore
Discover More Revision Notes Related to Catalysts to Deepen Your Understanding and Improve Your Mastery
