Photo AI
Last Updated Sep 26, 2025
The Atom & Radioactivity Simplified Revision Notes for Leaving Cert Physics
Revision notes with simplified explanations to understand The Atom & Radioactivity quickly and effectively.
416+ students studying
The Atom & Radioactivity
Ernest Rutherford and his team fired alpha particles at a very thin sheet of gold foil. The scattered particles were detected by tiny flashes of light on a fluorescent zinc sulphide screen.
- Most particles passed straight through the foil undeflected → showing that the atom is mainly empty space.
- Some particles were deflected at small angles → caused by passing close to a small, dense, positively charged region.
- A very small number of particles were deflected back at angles greater than 100° → indicating a collision with, or near, the nucleus itself.
From this, Rutherford concluded that the nucleus is extremely small, dense, and positively charged, and that the electrons orbit the nucleus in the mostly empty space around it.
Emissions Spectra
When a solid, liquid, or gas is heated or an electric current is passed through it, electrons in the atoms absorb energy and move to higher energy levels. When these excited electrons fall back down to lower levels, they release energy in the form of light.
If this light is passed through a prism or diffraction grating, it is dispersed into its component wavelengths, producing a spectrum. The exact pattern of coloured lines (the emission spectrum) is unique to each element, acting like a fingerprint for identifying substances.
Niels Bohr's Model of the Atom
- Electrons in an atom can only occupy certain fixed energy levels (orbitals).
- When an electron absorbs energy, it can jump to a higher orbital, moving into an excited state.
- This excited state is unstable and temporary. The electron soon falls back to a lower energy level.
- As it falls, it emits a photon of electromagnetic radiation with frequency fff.
- The energy of this photon corresponds to the difference between the two energy levels.
- Each possible electron transition has a definite energy difference, and so each photon has a definite frequency (or colour).
- This explains why elements produce line spectra: a unique set of discrete colours corresponding to specific electron transitions.
Radioactivity
- Here's a polished and complete version of your Radioactivity note, ready for Leaving Cert Physics revision:
Radioactivity
- Discovery: Radioactivity was first discovered in 1896 by Henri Becquerel, and further investigated by Marie and Pierre Curie, who identified new radioactive elements such as polonium and radium.
- Artificial Radioactive Isotopes:
- Normally stable, non-radioactive atoms can be made radioactive by bombarding them with neutrons.
- These man-made isotopes are widely used in medicine, industry, and research.
Applications of Radioactivity
- Medical Imaging
- Radioactive tracers (e.g. technetium-99m) are used to produce images of organs.
- Allows doctors to diagnose conditions without surgery.
- Medical Therapy
- High-energy radiation (e.g. cobalt-60) is used in radiotherapy to kill cancer cells.
- Food Irradiation
- Controlled doses of radiation kill bacteria, parasites, and pests.
- Helps preserve food and extend shelf life.
- Smoke Detectors
- Contain a small amount of americium-241, which emits alpha particles.
- Smoke disrupts the flow of ions, triggering the alarm.
Ionisation
- Ionisation occurs when radiation has enough energy to remove electrons from atoms or molecules, leaving behind charged ions.
- Alpha particles are strongly ionising because they are heavy and carry a double positive charge.
- Beta particles are moderately ionising, lighter and faster than alpha.
- Gamma rays are weakly ionising, but highly penetrating.
- Ionisation can damage living tissue, leading to burns, mutations, or cancer.
- Ionisation is the principle behind devices like Geiger–Müller tubes, which detect radiation by counting ionising events in a gas.
Geiger – Müller Tube
- Operates on the principle of ionisation
- Detects radiation, ionisation produced, activity of the sample.
- Radiation enters through thin mica window into the argon gas at low pressure.
- Ionisation of gas molecules produced electrons and positive gas ions
- Electrons accelerate towards the anode, colliding and ionizing more gas molecules to produce an avalanche of electrons
- The electrons reach the anode and are detected as a pulse.
Investigate range or identify radiation source
- Background rate: set counter to zero without any radiation around and record number of counts over 5-minute period and calculate number of counts per second.
- Place source of radiation in front of detector and find average count per second.
- Move detector away in small steps while continuously counting the number of counts per second until the count rate = background rate the distance from source = its range.
Greatest range = gamma radiation
Testing penetrative ability of sources
- Placing different materials between source and detector
To demonstrate ionizing effect of radioactivity
- Bring radioactive source close to cap of charged Gold Leaf Electroscope and the leaves will collapse
The radiation ionizes air molecules around the cap and the ions with opposite charge on the cap are attracted to the ions created around them. O.L.E neutralizes and leaf collapses.
Low-level radiation that everybody is expressed to every day.
Random Gas from granite lock is main source of background radiation
Effect of ionizing radiation on humans depends on:
- Cosmic rays
- Man-made radioactive materials
- Type of radiation
- Activity of the source
- Time of exposure
- Type of tissue irradiated
Precautions when using ionising radiations:
(Detect radiation by solid state Detector 100)
- Minimize time spent using proper protective / sources of radiation
- Use clothing
- Use tongs for handling sources
- Sources shielded away
Uses of Radioisotopes
Medical imaging – image of organ by placing short lived isotopes in it
Medical therapy – kill cancer sells easier than healthy
Food irradiation – gamma rays sterilize food
Carbon dating – determine activity of carbon-14 isotope in them
Alpha Particle
- Fast-moving helium nucleus emitted from nucleus of radioactive source
24HP
Parent nucleus → Daughter nucleus + Alpha - Particle
24X → Z - 2A - 4Y + 24He
Beta Particle
High-speed electron emitted from nucleus of radioactive atom, neutron splits into proton and electron, and e- is ejected at high velocity.
-1 neutron, +1 proton
ZAX → Z + 1AY + - 10e
Gamma Rays
High-frequency electromagnetic radiation emitted from nucleus of a radioactive atom.
Structure of nucleus remains the same it only loses energy to become more stable.
- Normally occurs after α or β emission
- Find change in mass no. [Only α - particles can affect that]
- Find change in atomic no [apply effect of α - particles to find β]
Activity
- The activity of a radioactive sample is the number of nuclei disintegrating per second.
- The SI unit of activity is the becquerel (Bq): 1 Bq = 1 disintegration per second.
- Radioactive decay is a random process:
- It is impossible to predict which nucleus will decay at any given time.
- However, for a large number of nuclei, the overall rate of decay follows predictable laws (exponential decay).
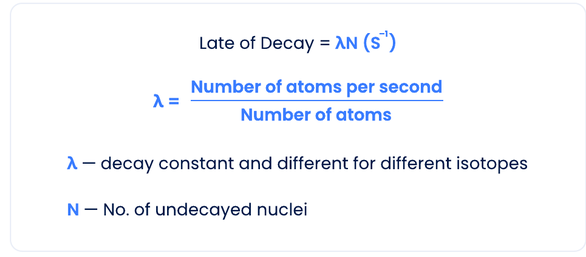
The Law of Radioactive Decay
The number of nuclei decaying per second is directly proportional to the number of undecayed nuclei.
Rate of Decay = λN (s⁻¹)
λ = Number of atoms per second / Number of atoms
λ — decay constant and different for different isotopes
N — Number of undecayed nuclei
- Activity
- No. of Particles undergoing decay per second
- Rate of Decay
- No. of disintegration per second
- No. of particles emitted per second
dN/dt
Half-Life
Time taken for half of the undecayed nuclei of the radioactive nuclei to decay
In one half-life : N decreases by half and so does rate of decay and so does the activity. To decay from 12 → 6 = To decay from 6 →
864s → T 1/2
8640s → 10 T 1/2
Relative mass ≈ mass number
Mole
Amount of a substance that contains as many particles as there are atom in 12g of carbon -12.
500K+ Students Use These Powerful Tools to Master The Atom & Radioactivity For their Leaving Cert Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
40 flashcards
Flashcards on The Atom & Radioactivity
Revise key concepts with interactive flashcards.
Try Physics Flashcards4 quizzes
Quizzes on The Atom & Radioactivity
Test your knowledge with fun and engaging quizzes.
Try Physics Quizzes30 questions
Exam questions on The Atom & Radioactivity
Boost your confidence with real exam questions.
Try Physics Questions3 exams created
Exam Builder on The Atom & Radioactivity
Create custom exams across topics for better practice!
Try Physics exam builder117 papers
Past Papers on The Atom & Radioactivity
Practice past papers to reinforce exam experience.
Try Physics Past PapersOther Revision Notes related to The Atom & Radioactivity you should explore
Discover More Revision Notes Related to The Atom & Radioactivity to Deepen Your Understanding and Improve Your Mastery
Load more notes