Photo AI
Last Updated Sep 27, 2025
Sample Answer for Measurement of the specific latent heat of fusion of ice
484+ students studying
Measurement of the specific latent heat of fusion of ice
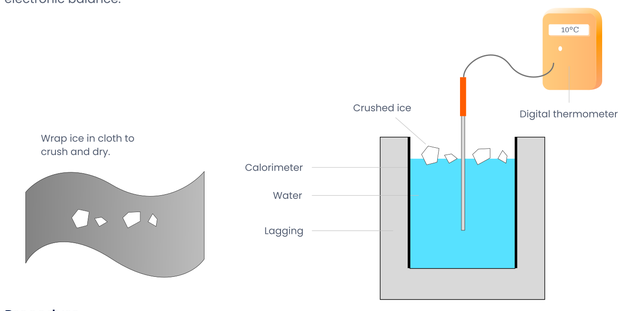
Apparatus
Ice, water, calorimeter, lagging, beakers, kitchen paper, digital thermometer reading to 0.1° C and electronic balance.

Procedure
-
Place some ice cubes in a beaker of water and keep taking the temperature with the thermometer until the ice-water mixture reaches 0° C.
-
Find the mass of the calorimeter .
-
Half fill the calorimeter with water warmed to approximately 10° C above room temperature. Find the combined mass of the calorimeter and water . The mass of the water is .
-
Record the initial temperature of the calorimeter plus water.
-
Surround the ice cubes with kitchen paper or a cloth and crush them between wooden blocks – dry them with the kitchen paper.
-
Add the pieces of dry crushed ice, a little at a time, to the calorimeter. Do this until the temperature of the water has fallen by about 20° C.
-
Record the lowest temperature of the calorimeter plus water plus melted ice. The rise in temperature of the ice is C and the fall in temperature of the calorimeter plus water is .
-
Find the mass of the calorimeter plus water plus melted ice m3. The mass of the melted ice is .
Measurement of the specific latent heat of fusion of ice
Physics Experiments
Results
| Measurement | Symbol | Value |
|---|---|---|
| Mass of the calorimeter | = | |
| Mass of the calorimeter plus the water | = | |
| Mass of the water | = = | |
| Initial temperature of the calorimeter plus water | = | |
| Final temperature of the calorimeter plus water plus melted ice | = | |
| Rise in temperature of the ice | = = | |
| Fall in temperature of the calorimeter plus water | = = | |
| Mass of the calorimeter plus water plus melted ice m3 = Mass of the melted ice | = | |
| = |
Calculations
Assume heat losses cancel heat gains. Given that the specific heat capacity of water cw and the specific heat capacity of copper cc are already known, the latent heat of fusion of ice l may be calculated from the following equation:
Energy gained by ice = energy lost by calorimeter + energy lost by the water.
If a polystyrene container is used in place of the copper calorimeter, the energy gained by the ice is equal to the energy lost by the water. The energy equation now reads:
To avoid melting the crushed ice, transfer it with a plastic spatula.
500K+ Students Use These Powerful Tools to Master Heat & Heat Transfer For their Leaving Cert Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
21 revision notes
Revision Notes on Heat & Heat Transfer
Revision notes with simplified explanations for multiple topics.
Try Physics Revision Notes40 flashcards
Flashcards on Heat & Heat Transfer
Revise key concepts with interactive flashcards.
Try Physics Flashcards4 quizzes
Quizzes on Heat & Heat Transfer
Test your knowledge with fun and engaging quizzes.
Try Physics Quizzes28 questions
Exam questions on Heat & Heat Transfer
Boost your confidence with real exam questions.
Try Physics Questions21 exams created
Exam Builder on Heat & Heat Transfer
Create custom exams across topics for better practice!
Try Physics exam builder117 papers
Past Papers on Heat & Heat Transfer
Practice past papers to reinforce exam experience.
Try Physics Past PapersOther Sample Answers related to Heat & Heat Transfer you should explore
Expand your writing skills with more engaging sample answers, covering vivid experiences, places, and unforgettable moments.
See Sample Answers from other students
See sample answers from other students, showcasing different writing styles and approaches to help you refine your own essays with clarity and creativity.
96%
114 rated
Heat & Heat Transfer
Understanding Heat Transfer in Different States
Dr. Jane Smith
187KViews96%
114 rated
Heat & Heat Transfer
Latent Heat: Definitions and Applications
Prof. John Doe
185KViews96%
114 rated
Heat & Heat Transfer
Thermal Properties of Water and Ice
Alice Johnson
185KViews96%
114 rated
Heat & Heat Transfer
Practical Applications of Latent Heat
Mark Thompson
192KViews