Photo AI
Last Updated Sep 27, 2025
Sample Answer for Measurement of specific heat capacity of water by an electrical method
350+ students studying
Measurement of specific heat capacity of water by an electrical method
Apparatus
Joulemeter, calorimeter, heating coil, beaker, lagging, thermometer reading to 0.1° C, electronic balance and a low voltage a.c. supply.
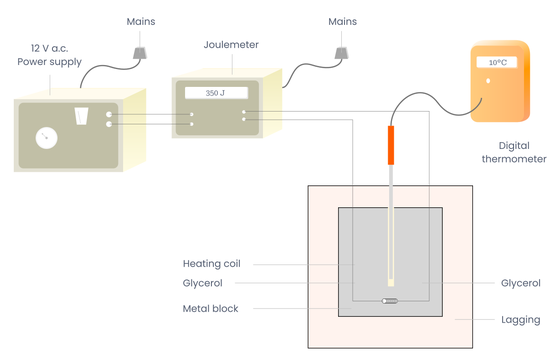
Procedure
-
Find the mass of the calorimeter .
-
Find the mass of the calorimeter plus the water . Hence the mass of the water is .
-
Set up the apparatus as shown Record the initial temperature .
-
Plug in the joulemeter, switch it on and zero it.
-
Switch on the power supply and allow current to flow until a temperature rise of 10° C has been achieved.
-
Switch off the power supply, stir the water well and record the highest temperature . Hence the rise in temperature is .
-
Record the final joulemeter reading Q.
Measurement of specific heat capacity of water by an electrical method
Results
Mass of the calorimeter: =
Mass of the calorimeter plus the water: =
Mass of the water: = =
Initial temperature of water: =
Final temperature: =
Rise in temperature: = =
Final joulemeter reading: =
Calculations
Given that the specific heat capacity of the calorimeter ccal is known, the specific heat capacity of water cw can be calculated from the following equation:
Electrical energy supplied = energy gained by water + energy gained by calorimeter
Given that the specific heat capacity of the calorimeter ccal is known, the specific heat capacity of water cw can be calculated from the following equation:
The energy equation now reads:
If a joulemeter is unavailable, electrical energy can be supplied to the heating coil from a power supply unit connected in series to an ammeter and rheostat. A voltmeter must be placed in parallel with the heating coil to measure the potential difference and a stopwatch used to measure the time of current flow.
If a calorimeter is used the energy equation is:
If a polystyrene container is used the energy equation is:
500K+ Students Use These Powerful Tools to Master Heat & Heat Transfer For their Leaving Cert Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
21 revision notes
Revision Notes on Heat & Heat Transfer
Revision notes with simplified explanations for multiple topics.
Try Physics Revision Notes40 flashcards
Flashcards on Heat & Heat Transfer
Revise key concepts with interactive flashcards.
Try Physics Flashcards4 quizzes
Quizzes on Heat & Heat Transfer
Test your knowledge with fun and engaging quizzes.
Try Physics Quizzes28 questions
Exam questions on Heat & Heat Transfer
Boost your confidence with real exam questions.
Try Physics Questions21 exams created
Exam Builder on Heat & Heat Transfer
Create custom exams across topics for better practice!
Try Physics exam builder117 papers
Past Papers on Heat & Heat Transfer
Practice past papers to reinforce exam experience.
Try Physics Past PapersOther Sample Answers related to Heat & Heat Transfer you should explore
Expand your writing skills with more engaging sample answers, covering vivid experiences, places, and unforgettable moments.
See Sample Answers from other students
See sample answers from other students, showcasing different writing styles and approaches to help you refine your own essays with clarity and creativity.
96%
114 rated
Heat & Heat Transfer
Understanding Heat Transfer Methods
Jane Doe
196KViews96%
114 rated
Heat & Heat Transfer
Thermal Properties of Water Explained
John Smith
190KViews96%
114 rated
Heat & Heat Transfer
Principles of Thermodynamics and Their Applications
Emily Johnson
192KViews96%
114 rated
Heat & Heat Transfer
Experimental Techniques in Heat Capacity Measurement
Michael Brown
197KViews