Photo AI
Last Updated Sep 24, 2025
Chemistry - Basic Elements Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Chemistry - Basic Elements quickly and effectively.
416+ students studying
Chemistry - Basic Elements
Definition of an Element
- Element: A fundamental chemical substance that cannot be broken down into simpler substances through chemical processes.
- Atoms in an element: have the same number of protons, a core concept for understanding elements.
All atoms within an element possess an identical number of protons. This is crucial for distinguishing elements from one another.
Historical Development of the Concept of Elements
Overview Table
| Era/Philosophy | Key Concepts |
|---|---|
| Ancient Greeks (e.g., Plato, Aristotle) | Classic elements like earth, water, air, fire |
| Alchemy | Pursuit of transformation and transmutation |
Ancient ideas were philosophical rather than scientific.
Historical Figures
- John Dalton: Introduced the atomic theory, proposing that elements comprise unique atoms.
- Dmitri Mendeleev (1869): Developed the periodic table, arranging elements by atomic mass.
Antoine Lavoisier's Contributions
- Antoine Lavoisier (late 18th century): A leader in modern chemistry, he emphasised the Law of Conservation of Mass.
- Lavoisier identified 33 elements and differentiated between metals and non-metals, forming the basis for contemporary chemistry.

Modern Definition and Advancements
The modern definition of an element is a pure chemical substance composed of a single type of atom, fundamental to all chemistry.
- Significant Advancements:
- John Dalton: Established atomic theory, a crucial development in understanding atoms and their interactions.
- Dmitri Mendeleev: Provided the periodic classification that organises elements based on properties and atomic mass.
Symbol and Nomenclature Standards
Standardisation Overview
- Standardising element symbols: Standardising element symbols is essential for scientific discussion.
- Role of IUPAC:
- The International Union of Pure and Applied Chemistry (IUPAC) ensures uniformity.
- Benefits of Standardisation:
- Establishes a universal language for scientists worldwide.
- Facilitates the exchange of scientific information without ambiguity.
- Enhances understanding across diverse cultures and languages.
Historical and Linguistic Origins of Symbols
- Symbol Origins:
- Many symbols derive from Latin or other languages, reflecting the element's discovery or historical use.
- Example: 'Au' for gold is derived from Latin 'Aurum'.
Potassium's symbol 'K' comes from 'Kalium', an Arabic term, illustrating the blend of scientific cultures.

IUPAC Naming Procedures
- Naming Overview:
- IUPAC employs a rigorous system for naming newly discovered elements.
- Case Study: Element 114:
- Named Flerovium (Fl) to honour the Flerov Laboratory of Nuclear Reactions.
- Reflects cultural and scientific recognition within the name.
Introduction to the Periodic Table
- Engaging Opening: How does the periodic table foresee the properties of elements?
This tool was crucial for predicting elements prior to their discovery. Did you know?
- Purpose and Overview: The periodic table is organised by atomic number and properties, allowing scientists to understand elemental relationships.
- Expanded Historical Context: The table's evolution underscores significant milestones, from early classification attempts to current refinements.
Structure of the Periodic Table
- Groups and Periods:
- Groups as Families: Consider groups as families sharing common characteristics.
- Real-world example: Group 1 metals react intensely with water, often explosively, underscoring their high reactivity.
- Trends Across Periods:
- Changes include increasing electronegativity and decreasing atomic size.
- Applications: Recognising these trends aids in predicting element reactivity.
- Groups as Families: Consider groups as families sharing common characteristics.
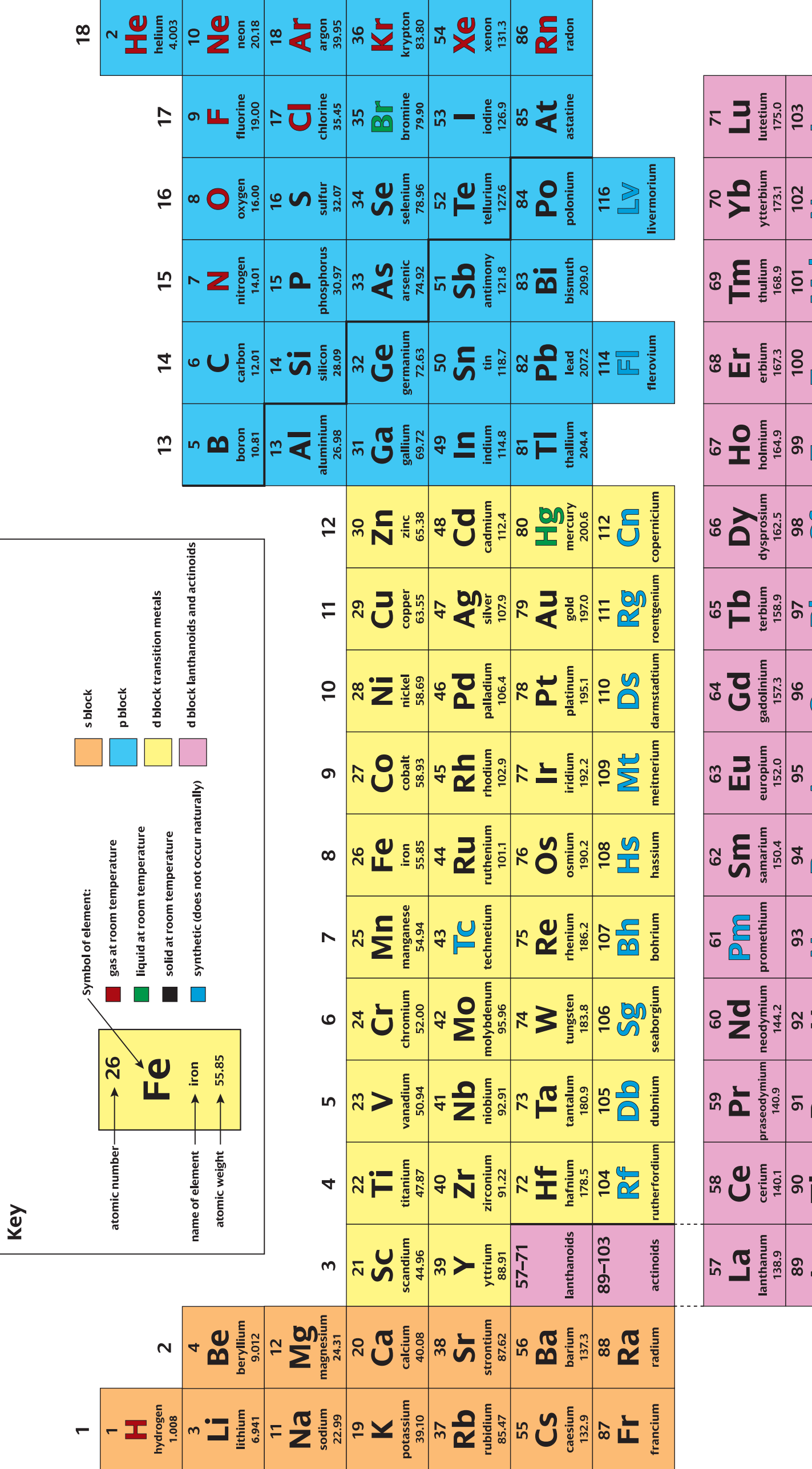
Historical Development
- Profiles of Key Figures:
- Dmitri Mendeleev: Predicted unknown elements through his innovative arrangement.
- Henry Moseley: Used atomic numbers to refine the organisation of the table.
Mendeleev's predictions were later validated, demonstrating the table's early success in scientific communities.
Classification of Elements
Definition of Categories
- Metals: Known for high electrical conductivity, lustre, ductility, and malleability.
- Examples: Iron, Copper, Aluminium.
Gold is lauded for both its exceptional malleability and conductivity.
- Non-Metals: Usually exhibit poor conductivity, low lustre, and can exist in various states (solid, liquid, gas).
- Examples: Carbon, Sulphur, Oxygen.
Bromine is a rare non-metal that is liquid at room temperature.
- Metalloids: Possess properties intermediate between metals and non-metals, crucial for their semi-conductive capabilities in technology.
- Examples include Silicon and Arsenic.
Silicon's importance as a semiconductor is vital for technological applications.
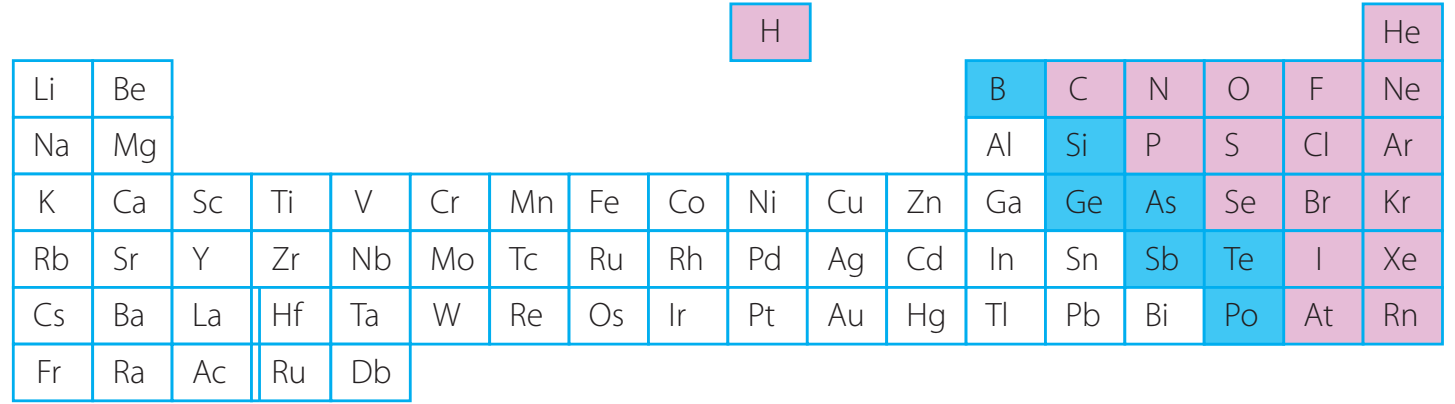
Key Properties of Elements
- Atomic Radius:
- Definition: The distance from the nucleus to the outermost electron.
- Trend Across Periods:
- Typically decreases.
- Reason: Increasing nuclear charge.
- Trend Within Groups:
- Increases down a group.
- Reason: Addition of electron shells.
Atomic Radius: Comprehending atomic radius aids in predicting bonding and reactivity of elements.
- Electronegativity:
- Definition: Reflects an atom's ability to attract electrons within a bond.
- Trend Across Periods:
- Increases across a period.
- Reason: Stronger nuclear charge.
- Trend Within Groups:
- Decreases down a group.
- Reason: Increased atomic radius and electron shielding.
Electronegativity: A higher electronegativity leads to stronger chemical bonds, influencing compound properties.
- Ionisation Energy:
- Definition: The energy required to remove an electron from an atom.
- Trend Across Periods:
- Generally increases.
- Reason: Stronger attraction between the nucleus and outer electrons.
- Trend Down Groups:
- Decreases down a group.
- Reason: Larger radii and more electron shielding.
- The anomaly between nitrogen and oxygen is due to electron pair repulsion in oxygen, lowering the energy needed to remove an electron.
Ionisation Energy: Key for predicting an element's tendency to form positive ions.
500K+ Students Use These Powerful Tools to Master Chemistry - Basic Elements For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
442 flashcards
Flashcards on Chemistry - Basic Elements
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards40 quizzes
Quizzes on Chemistry - Basic Elements
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes7 questions
Exam questions on Chemistry - Basic Elements
Boost your confidence with real exam questions.
Try Chemistry Questions2 exams created
Exam Builder on Chemistry - Basic Elements
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Chemistry - Basic Elements
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Chemistry - Basic Elements you should explore
Discover More Revision Notes Related to Chemistry - Basic Elements to Deepen Your Understanding and Improve Your Mastery
